
Leaders in Plastic Surgery
Skip other details (including permanent urls, DOI, citation information): This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License. Please contact [email protected] to use this work in a way not covered by the license.
For more information, read Michigan Publishing's access and usage policy.
CHAPTER SEVEN: Clinical Research, Clinical Procedures, New Techniques—1946–86
After discussion among the authors, editors, and contributors to this book, it was decided to describe as succinctly as possible the clinical activities of Drs. Dingman (ROD) and Grabb (WCG) leading up to 1964. Beyond that point, we decided to provide a sampling of the expanding clinical activities by categories rather than solely in historical order. In addition, we asked our colleagues Drs. Markley and Austad, as well as Dr. Hack Newman, to provide their personal recollections of three important clinical areas that contributed to define this highly innovative and transformative era of plastic surgery history: replantation and free tissue transfer, clinical use of expansion, and the development of craniofacial surgery at the University of Michigan (UM).
Early Work of Dr. Dingman and Dr. Grabb
Prior to his plastic surgery training with Dr. Ferris Smith that was completed in 1946, Dr. Dingman’s curriculum vitae (CV) indicated his clinical activity was mostly limited to oral surgery conditions and concerns. One exception was an article in 1939 in the Archives of Otolaryngology about periocular sinuses. The other published subjects include management of facial fractures, osteomyelitis and tumors of the jaws, abnormalities of the temporomandibular joint (TMJ), and orthognathic surgery. However, beginning in 1948 and up until Dr. Grabb joined him in 1961, Dr. Dingman’s emphasis shifted to subjects more of an interest to plastic surgeons. In 1948, he published an article on surgical correction of developmental deformities of the mandible in the second volume of the Plastic and Reconstructive Surgery (PRS) journal.[1] This was followed by articles on such varied subjects as cleft lip,[2] iliac bone grafts,[3] malunion of facial fractures,[4] Z-plasty,[5] radiated costal cartilage,[6] ostectomy of the mandible in cleft lip and cleft palate patients,[7] and rhinoplasty caused by defects of the septum.[8] In 1961, when Dr. Grabb was a resident and coauthor, two articles were published, one on lymphangioma of the tongue[9] and the other on human rib cartilage grafts preserved by irradiation.
The latter article turned out to be an important contribution to facial reconstruction. The following is quoted from the introduction to the 1961 PRS article by Dingman and Grabb[10]: “Preserved costal cartilage homografts have proven to be of value in restoring contour defects of the supporting structures of the face. The cartilage can be sterilized and preserved indefinitely. It can be easily sculptured at the operating table and after implantation it is well tolerated by the body tissue.” Dr. Dingman quoted an earlier article from 1956,[11] where he and two others reported the use of irradiation to sterilize canine costal cartilage, and it was subsequently determined that sterilization of human costal cartilage could be accomplished by rapid (fifteen hours) cobalt 69 gamma irradiation using 3,000,000 rep. This was carried out in the Ford Nuclear Reactor in the Phoenix Memorial Laboratory at the UM.
The cartilage was obtained at autopsy, ideally from young adults, and was refrigerated and stored until it could be prepared by a resident assigned to this project. The cartilage was stripped of soft tissues and the perichondrium was preserved. It was then cut into lengths to fit into a glass canning jar filled with saline and tightly sealed. After sterilization by the irradiation, the cartilage is ready for use the next day or can be stored indefinitely. The article reported that twenty-eight of thirty human patients receiving these grafts for chin augmentation, orbital floor defects, and dorsum of the nose showed no clinical evidence of absorption in seven months to 3½ years postoperatively. (The two that absorbed were used for external ear reconstruction where autologous cartilage is essential for success.) This knowledge of the successful utilization of homograft cartilage was essential in our reconstructive efforts as there were few other materials available or more dependable for many years after this report. We all kept a few of these jars containing the irradiated cartilage close at hand in the operating room (OR) suite for many years. Illustrative of Dr. Dingman’s intellectual generosity is the statement in the summary in the 1961 article that the arrangements for the resources of the Phoenix lab at the UM would be available for others interested in adopting this technique.[12] Dr. Wexler sent his recollection about the cartilage collection (which has undergone some minor editing):
Twice a month we went to the morgue, opened chests, cutting the out cartilaginous part of the ribs [cutting ribs in to 4 cm lengths], then put them into saline-filled transparent glass jars. They were taken to the atomic reactor on North Campus, irradiated with 3 million rads and the jars became brown. We kept the jars on the shelves ready to be used as filling material—similar to the silicones today. This was excellent, curvable, infection resistant material. One evening, in the winter, I took a bar [of cartilage] to be used at Jackson prison. I left it in my car. Next morning I found a block of ice in the car surrounded with broken glass. What happened to the cartilage? I do not recall exactly. Why might it have been taken and what to be used for?[13]
Paul Izenberg recalled six years later that the cadaver cartilage was still being collected: “I remember being called into the section office by Lauralee and being told ‘it’s time’ knowing that the task of harvesting cartilage for processing was upon us. So a couple of us (usually junior residents) hiked over the cadaver lab to process the specimens.”[14] Paul suggests that this denatured homograft material was, in a way, a forerunner of acellular dermal matrix, now one of the most often used homografts in plastic surgery.[15]
In further review of Dr. Dingman’s CV, it was noted that starting around the time in 1952 when he turned down the chairmanship of oral surgery at the dental school and aligned himself with the surgery department at the medical school, he had published a series of articles during the subsequent ten years, either collaborating with someone from another specialty in the medical center or on a subject only peripherally related to plastic surgery but published in one of the other specialty journals. Examples include an article about Z-plasty to the urethral meatus, published with Dr. Reed Nesbit who was chairman of the urology department; an article about cheilitis glandularis with reconstruction of the upper lip, with Dr. Arthur “Whitey” Curtis who was head of the dermatology department; and an article about semi-open burn management, with Dr. Irving Feller, a member of the general surgery section and on his way to becoming a burn specialist who was responsible for setting up the Burn Unit at UM. Examples of the second category include an article about necrobiosis lipoidica diabeticorum published in AMA Archives of Dermatology and Syphilology 1951,[16] one regarding a malunion of the zygoma in the Transactions of the American Academy of Ophthalmology,[17] and another about a burn scar contracture of the neck in the Surgery Clinics of North America. While noting the subjects and coauthors of these various publications, it occurred to me that perhaps during those ten or so years, Dr. Dingman was making a concerted effort to reach out and show the applicability of basic plastic surgery principles in the management of a wide variety of medical and surgical problems. He was also trying to heighten awareness of the value of having plastic surgery as a readily available consulting service within a major medical center. Perhaps just as important were his efforts toward building rapport among the medical staff to gain support for the ultimate formation of a plastic surgery section in the medical center within the Department of Surgery.
Subsequently, the range of continuing and varied interests, as shown in his list of publications, continued to expand rapidly. Some of the subjects included management of tumors, wound healing, and Z-plasty. A particular interest involved facial injuries associated with auto accidents. This led to a series of articles from 1960 to 1968, published together with Dr. Grabb and Dr. Donald Huelke, a professor in the anatomy department and a research scientist at the UM Transportation Research Institute. The subjects included facial injuries due to windshield impacts injuries and deaths from windshield and instrument panel impacts. One of the most significant findings, published in 1968, concerned the decrease in frequency and severity of facial lacerations after introduction of the new automobile windshield design.[18] This new design almost single-handedly eradicated the frequent and extensive facial lacerations (one to three patients a week seen in our emergency rooms) that resulted from the vehicle’s occupant’s head penetrating the older design windshields in front-end collisions (no seat belts in those days).
Along the way, new instruments were introduced. The first was a bone and cartilage grasping forceps in 1954[19] that became widely used. The next was a newly designed plastic surgery dressing cart for use in hospitals and modifiable for use in other specialties. The details of the design were published in 1957 with coauthors Dr. Paul Natvig, who was still a plastic surgery resident, and Jim Winkler, who was then a resident in general surgery at Saint Joseph Mercy Hospital (SJMH).[20] In 1965, with Dr. Grabb as coauthor, Dr. Dingman introduced the now universally used Dingman mouth gag for cleft palate repair in 1962 (discussed, with photo, on pp. 99–100).[21] He also developed the double-ended Dingman periosteal elevator, the Dingman ear abrader (described under the discussion of otoplasty, p. 107), and the transaxillary augmentation mammoplasty dissector (described in the section on augmentation mammoplasty, p. 90).
It is difficult to summarize the enormous clinical output of the next several years. Dr. Dingman’s interest in innovations and clinical problems encompassed a wide range of subjects in addition to those already mentioned: facial reconstruction; face, brow, and eyelid surgery; rhinoplasty; otoplasty; scalp and lip reconstruction; cleft lip repair; other developmental abnormalities; chest wall reconstruction; treatment of multiple types of benign and malignant lesions; and TMJ surgery.
In 1964, there was an important article on TMJ reconstruction with metatarsal grafts by Dr. Dingman with Dr. Grabb as coauthor.[22] The article reported and described the technique Dr. Dingman developed for treating a twenty-nine-year-old female to correct the deformity resulting from a previous operation, ten years earlier, when a surgeon had removed her bilateral mandibular condyles as a radical method to treat intractable TMJ symptoms. On presentation, she had a severe open bite with only her molar teeth in occlusion. Restoration of the condyles was accomplished by using the heads of the bilateral fifth metatarsal bones as fresh autogenous grafts. The patient was followed for seventeen months, and x-rays showed transplanted bone to be intact, and normal occlusion and mandibular function restored, with markedly improved speech. She had no complaints with her feet and was wearing only a slightly narrower shoe than before. The authors drew the conclusion that half-joint transplants do not undergo the destructive changes of bone and cartilage that occur in whole joint transplants. This was a very significant finding. Interestingly, several months later, the patient had an irradiated costal cartilage graft to correct her retruding chin deformity. A 1975 article (referenced and discussed in p. 114) reported an additional eight more cases using metatarsal grafts in a variety of deformities of the TMJ, all with satisfactory results.
There were several articles published and many coauthored with Dr. Grabb on various aspects of both acute traumatic and secondary deformities of facial bones. Of course, the highpoint of the published work on that subject was Drs. Dingman and Natvig’s book titled Surgery of Facial Fractures, published in 1964 (Photo 52). It became the “bible” for the treatment of facial fractures (referenced and discussed in p. 112). Dr. Dingman always encouraged and invited both his colleagues and residents to join him in these many projects that resulted in publications. That continued well beyond his retirement as section head.
In those early years, in addition to Dr. Grabb and myself, there were faculty members from many disciplines as coauthors with Dr. Dingman. These included Dr. Harlan Bloomer, director of the speech clinic; Dr. Donald Huelke; Gerald Hodge, head of medical illustration; and Dr. Robert Ponitz, DDS, an orthodontist in private practice who worked closely with all of us in our orthognathic cases as well as consulting in the SJMH Cleft Palate Clinic. The list also includes Krystyna Pasyk, PhD, who was an active participant for many years in the Sargent laboratory, and Dr. Gary Sandall, a pediatric ophthalmologist. Examples from the resident/fellow group of coauthors through these years and beyond include James Winkler; Jim Bennett; Bob Knode; Ralph Seaton; John Markley, who was a student at the time; Eric Constant; Don Davis; Grant Fairbanks; Ron Wexler; Joe Agris; Bob Wilensky; Isaac Peled; Paul Izenberg; Paul Dempsey; Paul Natvig; Bob Gilman; and Malcolm Marks.
When Dr. Grabb completed his residency, he joined Dr. Dingman in practice at SJMH. From the very beginning, he brought innumerable ideas and much energy to the academic side of the residency training program. One example is the clinical study of the anatomy of the mandibular ramus of the seventh nerve published together with Dr. Dingman in 1962.[23] One hundred human facial halves were dissected with cooperation from Dr. Russell Woodburn, head of the Department of Anatomy. Dr. Woodburn was a wonderful and inspiring teacher and friend to all surgeons who were interested in the details of anatomy. This study, carried out on embalmed cadavers, defined that the position of the mandibular ramus, anterior to the facial artery, was always above the lower border of the mandible. At the posterior, the branch or branches were often below the mandibular border, and this was a warning to the surgeon approaching this area to undertake an open reduction of the mandible. This study was well received and referred to often in the OR.
Breaking new ground, five years later, Dr. Grabb published an important study that described and clarified the characteristics of the first and second branchial arch syndrome.[24] This syndrome was defined as a constellation of abnormalities resulting in a spectrum of facial malformations that blend with one another and have no bold or clear lines of delineation. The paper was based on a study of 102 patients who were identified by birth records at SJMH and who agreed to return for clinical evaluation. This was a pioneering study and very well regarded at the time. It was an important step leading to a new and clearer idea of a “sequence,” which defines the spectrum of anomalies found within types of congenital facial deformities.
As mentioned in detail under his tenure as section head and so beautifully stated in Dr. Dingman’s eulogy, Bill Grabb was a prolific researcher, writer, and teacher in addition to being an inspirational and highly respected leader.
Categories of Clinical Activities
Hand Surgery
Up to 1974, Bill Grabb, John Tipton (until 1969), and I managed the majority of soft tissue injuries of the hand and forearm at SJMH. Most of the hand trauma in that period at the UM was taken care of on the orthopedic service unless directly referred to plastic surgery. A lot of upper extremity tendon and nerve injuries, as well as some quite serious soft tissue trauma and burns, came under our care, which nicely augmented the experience our residents were having on the Detroit Hand Service rotation. In 1967, ROD, WCG, and I published in the Michigan State medical journal, an update on current use of flexor tendon grafts.[25] Later, because of the common postgraft tendon adhesion that occurred in the more complex injuries and limited tendon excursion, we began to use, in badly traumatized injuries, the “Hunter silicone rod” technique. These rods were placed into the palm and fingers to stimulate a new tendon sheath with less chance of forming adhesions after the tendon graft was placed in a secondary procedure.
At SJMH, we usually collaborated with orthopedic consultants when there were associated bony injuries. They were happy to share in the tendon repair work after there was an accidental medial nerve stripping on their service in an attempt to procure a palmaris longus graft. One of the cases I did early in my practice illustrated the principle of sharing part of a nonfunctional digit to restore function in an essential digit. The patient had a partial amputation of his dominant thumb just distal to the M-P joint and of his adjacent index finger through the mid-proximal phalanx. This injury resulted in a nonfunctional, too-short thumb and a nonuseful index finger stump that was in the way. A neurovascular transfer of the residual index finger phalanx to lengthen the residual thumb and a ray amputation of the second metacarpal restored sensate thumb function for pinch and grasp and eliminated the in-the-way index digit. That case made me realize I had made the right choice in becoming a plastic surgeon.
John Markley provided his recollections of the hand program at the UM from 1974 to 1984:
I was interviewed by Dean Louis in May 1974 as part of the process of my appointment to start in July 1974, at which time I suggested forming a combined Plastic Surgery-Orthopedic Surgery hand service. He was not at all interested, and we agreed to co-exist amicably and compete. At that time orthopedics did not have any microsurgical capability.
When I started in 1974, I was named director of the Plastic Surgery Hand Service, and started a one-afternoon-a-week hand clinic, staffed by me, with one resident (sometimes two, a junior and a senior), and Loraine Smith, one of our nursing aides [Photo 53]. This started out slowly but it gradually built, and had a big boost with the first digital replant I did in 1976 (first in Michigan). Numerous replant cases followed. In addition, I did the first two microsurgical free toe-to-hand transfers in 1978 and 1979 with the residents at that time. However, beyond the microsurgery, the overall hand experience grew nicely. I lobbied and educated other specialties, for example, pediatrics, neurology, rheumatology, emergency medicine, and so on, and cases came in from all those and continued until I left in July 1983. Between then and 1984, I continued to see problem hand cases once a month in the UM Hand Clinic on a consultant basis for cases selected by Dr. Tom Stevenson.
I started the annual hand/forearm gross anatomy dissection series, three 1.5 hour dissections each fall in 1980 and continued that every year [until I retired in 2007].[26]
This is another excellent example of the great teaching value created by the availability of fresh cadaver anatomy specimens. (There is now a videotape of one of his dissections in the residents’ online teaching library.)
Markley continued,
The part of the timeline that I cannot fully recall, nor do I have any records to help, is this. When I started in 1974, the residents were still going to Detroit for additional hand experience. That did not stop until I had been doing the hand clinic for a while (probably about 1982). In addition, at some point in the 1976–1978 time frame, I started teaching the residents micro-neurovascular suture technique using rats in the Kresge [Sargent] lab. However, somewhere in that time I advised sending the residents to Louisville for Bob Acland’s week-long microsurgical courses and that was done, I think until 1983–84.
Markley continued, discussing hand joint replacement with silicone implants:
The metacarpal-phalangeal (MPJ) implants and the interphalangeal joint (IPJ) implants are identical, just different sizes. I’m not sure when I first used the smaller ones in IPJs but likely within a couple years after 1976. The thing is, the indications for use in metacarpal and interphalangeal joints are all different somewhat, and less common in the IPJs than in MPJs. In the MPJs, the indication is nearly always rheumatoid, occasionally trauma or degenerative. They are rarely indicated in the DIJs where usually fusion is better. They work very well in individual PIJs with post-traumatic or osteoarthritis, under some circumstances, and I used them there much more than in DIJs but less frequently than MPJs. Use in IPJs is more technically demanding to get a good result since the silastic does bend laterally as well as dorso-volar, which is not a problem in MPJs but requires careful attention to ligamentous lateral stability in IPJs.[27]
One contribution John did not mention was the excellent PRS article he published in 1977 describing digital neurovascular island flaps and the discussion of two-point discrimination achieved.[28] In addition, I am a personal recipient of John Markley’s skill by virtue of an indicis proprius tendon transfer to repair a spontaneous ruptured extensor pollicis longus on my dominant hand in 1975 that to this day is functioning perfectly.
Treatment of Skin Cancers
In 1969, in a report in the UM medical journal from members of the Section of Plastic Surgery, the authors presented a statistical evaluation of treatment of basal cell and squamous cell carcinoma in 653 UM patients treated through 1961, thus allowing a five-year follow-up period.[29] Of great significance was the opportunity to use the university’s IBM 7090 computer, which allowed the analysis of the effect of a large number of factors on the cure rates and specifically the variables and treatments that were related to recurrences. Following are two of the most significant conclusions: significance of the specific type of treatment-affected cure rates was at the .05 confidence level and electrodessication and curettage was the most effective treatment and radiation therapy the least in producing cure rates. Location of tumors most likely to recur included lateral nose, internal nares, and external auditory canal. Ulceration-invasive growth pattern and multicentric appearance were also prominent factors for recurrence. The final comments of the authors included, “It is likely that methods of treating skin cancers have improved since 1961, when our present study ended.” It was thought that more effective treatment had been brought about by the following factors: (1) the use of Mohs’s chemosurgical method (Dr. Mohs reported a 0.9 percent recurrence rate in four thousand cases using his technique in a personal communication to the lead author), (2) all radiation therapy was now being given in a new unit by highly improved techniques, (3) most surgical treatments for skin cancers were now handled in the Section of Plastic Surgery. There were three important aspects of this study: an advanced computer was used for analysis; authorship included a medical student (Jesselson) and a plastic surgery resident (Dr. Seaton), an important factor in their education; and results of the study positively affected the subsequent management of these types of lesions. It was clear that complete excision of the lesion at the time of primary operation was essential to lowering the recurrence rate. To achieve this would require close collaboration with a clinical pathologist at the time of primary excision. In addition, the use of the Mohs technique should be strongly considered for specific difficult lesions.
We all saw patients with parts of their faces eaten away as a result of inadequately treated basal cell cancers. Thus, we were always looking for the most effective way of treating these lesions primarily. Our first line of action was a close collaboration at the time of initial excision with our pathologists at SJMH. We would do the initial excision with an adequate visual margin. Before completely removing the lesion, we would mark the specimen with a suture at one edge for orientation. Then we would take the specimen over to the pathology department and, together with the pathologist, decide the technique that would best determine whether the margins were clear. If not clear on initial exam, we would go back and re-excise the exact spot in question. Eventually, we would be told that the excision was complete and that we would either directly close the defect or use an appropriate reconstructive technique. This routine resulted in greatly minimizing recurrences, and we also learned a lot about skin pathology in the process. We were fortunate to have such cooperative colleagues.
However, there were types of basal cell lesions, such as morpheaform type, which were extremely bothersome because of their indistinct borders and their propensity to spread with subdermal extension. We were especially concerned about those lesions near the base of the nasal ala or medial canthus that might be tracking along a deeper plane. This is where Mohs’s surgery became very useful. Dr. Mohs, a surgeon at the University of Wisconsin, proposed and practiced a method of tissue fixation (with zinc chloride paste). He then removed the fixed specimen, mapping the entire specimen and sectioning in such a way as to determine the adequacy of excision. Dr. William Taylor, a professor of dermatology at UM and a close friend of Dr. Dingman’s, became quite a proponent of this technique. A training program was begun in dermatology, and soon, there were more Mohs surgeons available. In the early days, the procedure often took more than one day, which was a disadvantage for the patients. Eventually, the fresh technique evolved that allowed multiple sections to be examined in a single day. When the completed Mohs procedure provided assurance that the excision was complete, the surgeon could proceed with reconstruction without fear of camouflaging a recurrence and minimizing morbidity time for the patient.
Treatment of Cutaneous Vascular Lesions
In 1977, Dr. Grabb, with Dr. Malcolm (Biff) MacCollum (1973–75) and Nick Tan, published in PRS the UM’s experience with the use dermal tattooing in camouflaging cutaneous hemangiomas (port-wine stains).[30] An attempt was made to reaffirm successful results in a previous study from Toronto. Nineteen children up to sixteen years of age who had tattooing of the congenital port-wine hemangiomas were included in the report. A careful attempt was made to match the tattoo pigment with individual skin tones of each patient. This effort was supervised by Nick Tan, from medical illustration, who had training in preparing and mixing these pigments. None of the patients had ultimate improvement beyond 50 percent after at least two and sometimes five treatments. The consensus of the authors was that this technique was not effective enough in this age group of patients, due in part to gradual leaching out of the pigment.
In 1984, Marks, Argenta, and ROD presented five cases of traumatic arteriovenous malformations of the external carotid system.[31] In the cases presented, the lesions were characterized as containing multiple endothelial-lined channels between the arterial and venous system. The etiology was discussed. Accurate diagnosis is important and often can be made by feeling a thrill on palpation or a bruit on auscultation, and by Doppler study. Unless the lesion is small and well localized, its extent and the specific feeder vessels are best evaluated by arteriography. Four of the five cases presented confirmed by history that inadequate excision leads to recurrence and perhaps significant cosmetic deformity. Complete excision and ligation of all feeder arterial vessels is essential, and long-term follow-up in these five cases revealed no recurrence. Significant defects can result from excision that may require reconstruction. A thorough description of the cases was provided along with an excellent review of the literature. In that same year, Krystyna Pasyk, MD; Dingman; Argenta; and Gary Sandall (pediatric ophthalmologist) reported on eyelid and orbital capillary hemangiomas.[32] Congenital capillary (strawberry) hemangiomas are usually present at birth or shortly thereafter. They go through a growth phase and ultimately spontaneously regress or disappear starting by age five or six. However, those that involve the orbital area and obstruct the vision can result in a variety of ocular problems including loss of vision (amblyopia). The article stressed the critical importance of close coordinated follow-up by a pediatrician, ophthalmologist, and plastic surgeon. Treatment must be started aggressively if the lesion enlarges enough to even partially obstruct the infant’s vision. If the visual field becomes totally obstructed, intervention must be carried out within one week to prevent amblyopia. Initially, injectable steroids can be tried, but if not effective in quickly shrinking the tumor, surgical excision must be done. Because these lesions do spontaneously regress, total extirpation is not indicated. Clearing the field of vision is the primary objective.
Skin Flaps
Beginning in 1966, there was a concerted effort both in the laboratory and clinically to study how to improve the efficiency and utility of skin flaps to close wounds. The struggle to successfully close difficult wounds was an ongoing problem for all of us. We were motivated by clinical necessity to find ways to enhance the blood supply to random skin flaps and to find new ways to approach this problem. Time was spent in studying the delay phenomena and exploring various ideas to enhance the vascularity of both random and axial patterned flaps. Examples are the research projects that fellow resident Bob Knode, Bill Grabb, and I contributed to in the VA laboratory in 1966–67.[33],[34] Although we were optimistic at the onset, the results unfortunately showed that IV dextran did not enhance the vascularity of either variety of flap in the pig.
Many of the types of flaps available clinically to us at the time were cumbersome and not always successful. I think back to the necessity of applying cross-leg flaps to avulsion injuries of the opposite lower leg and the morbidity from the necessary immobilization. This was described very well in a paper by Grabb, Don Greer, and Bob Wilensky[35] along with Don Greer’s chapter in Grabb and Myers’ book on flaps.[36]
Dr. Grabb’s interests in skin flaps were wide-ranging and sustained. He became familiar with all the centers and surgeons who were working in this extensive and fascinating field of endeavor. The effort culminated in the publication of the book Skin Flaps[37] in 1975 that Dr. Grabb edited with Dr. Bert Myers, professor of surgery at Louisiana State University (LSU) and a tireless collaborator in flap research. Dr. Frank McDowell, the editor-in-chief of PRS, wrote the foreword for the book. A quote from the foreword clearly stated the book’s importance: “For too long we have not had a book devoted to all aspects of all kinds of flaps. To obtain such information one had to read parts of many books and many papers. Now it is all available to us in a compact volume, both experimental and clinical, written by the leading workers in each phase in the United States and abroad. Herein will be the answer to most of our questions about flaps. And from this we will get ideas for new uses of flaps and a better ways of moving flaps. How fortunate we are.”[38] Quotes from the preface, written by Drs. Grabb and Myers, clearly delineated the up-to-date status of the research and clinical development in the field of skin flaps as of the time of publication: “The principles that have been used in planning a skin flap were developed empirically over a period of centuries. In the past decade great advances as a result have permitted us to proceed with more confidence of success in the clinical use of skin flaps. These advances have been the stimulus for this book.”[39] There were two major divisions in the text. The first ten chapters pertained to some of the recent research on skin flaps. These chapters included flap circulation as well as effects of anesthetic agents and hyperbaric oxygen. The mechanism of the delay phenomenon, including the role of arteriovenous connections, was also discussed. A summary of the anatomy of cutaneous circulation was also presented. Pioneering authors included, among others, John Reinisch, Rollin Daniel, and Bert Myers, and references to Stuart Milton, a flap research pioneer, who had recently died and to whom the book was dedicated, were also present in the book. He was a close friend of Bill Grabb’s and a welcome visitor in Ann Arbor. He was initially responsible for questioning the validity of the absoluteness of length-width ratio in skin flap design.[40]
Continuing from the preface,
The remaining 31 chapters are devoted to the clinical aspects of skin flaps. In the clinical use of skin flaps, we are moving toward an atlas of safe skin flaps, but there is no rule or formula by which we can design a flap with assurance that it will survive. Rather we must rely on our accumulated experience to know that a flap with its base in a certain place when it’s designed to be of so many centimeters in length and a certain number of centimeters in width. In Part II we have asked the contributing authors to orient descriptions of specific flaps according to anatomic landmarks and provide, in centimeters, the dimensions of length and width that, in their experience would assure a viable flap in the healthy adult.[41]
Part II was an inclusive group of chapters providing a clear description of all the known flaps available for defects, literally from head to toe, written by experts recruited by the editors from around the world who had actually used these flaps. It was clearly a masterful accomplishment for its time, and it was the model for the book Encyclopedia of Flaps, which Dr. Grabb was working on at the time of his death in 1982. It was published posthumously in 1990[42] and is now in three volumes.
More from the Grabb and Myers preface: “The early determination of blood supply to random pattern and axial pattern flaps deserve emphasis and it was explained that in the clinical situation, one can accurately determine the adequacy of blood supply to both random and axial pattern flaps by the intravenous injection of fluorescein followed by a visualization of the fluorescence under an ultra violet lamp. In an axial pattern flap, a hand-held Doppler flowmeter can help locate and then monitor blood flow in the artery supplying the flap.”[43] Some of this experimental work was carried out at UM in clinical studies by Dr. Grabb with Dr. Sigurdur (Siggy) E. Thorvaldsson (1971–73), published in 1974.[44] It was a report of nineteen patients and showed that the use of IV fluorescein at the time of operation was a reliable and safe method for predicting how much of a skin flap would survive after raising it from its normal anatomic location. The results of this study and others as discussed by Dr. Bert Myers, in chapter one in the book Skin Flaps, gave us confidence in the reliability of the technique. Based on this study, we began to intraoperatively excise portions of skin flaps that failed to fluoresce. We followed this procedure both with reconstructive flaps and surgical ones such as in prophylactic mastectomies. This technique was a precursor to the currently used “SPY” intraoperative flap monitor.[45] Grabb and Myers’ book, Skin Flaps, was the bridge between the era of the pioneering understanding of random and axial flaps and the widespread use of musculocutaneous and fasciocutaneous flaps in the coming years.
Myocutaneous (Musculocutaneous) Flaps
The next dramatic steps with understanding flaps were recounted by Leonard Furlow, who with John McCraw coauthored the last chapter describing the dorsalis pedis arterial flap in Grabb and Myers’ book. In a 2014 editorial posted in the Annals of Plastic Surgery, titled “When Our Vocabulary Changed,”[46] Dr. Furlow explained that before the mid-1970s, the language we plastic surgeons used when we talked about flaps was geometric—length-width ratio, advancement, transposition—or anatomic—cross-leg, abdominal, or temporal, delayed or staged. Flaps, moved in a random network of vessels, often required delay or staging to improve the blood supply or to induce the flap to survive on a reduced blood supply. The few axial flaps previously identified, such as the forehead flap, deltopectoral flap, and neurovascular island flaps in the hand, had very circumscribed uses. The very innovative Dr. Miguel Orticochea reported constructing a penis using a muscle carried on its axial vessels with the overlying skin supplied by perforating vessels from the muscle. Stimulated by that specific solution, John McCraw saw the tremendous potential in understanding and describing a general myocutaneous pattern for the solution of a wide range of reconstructive challenges. When at Emory University, under Dr. Jurkiewicz, Dr. McCraw began defining by dissection a variety of these flaps and, with that knowledge, produced a virtual revolution that changed the field of reconstructive surgery forever.[47] Ultimately, this led Drs. McCraw and P. G. Arnold to begin an annual flap dissection course in 1977 in Norfolk, Virginia, and ultimately to publish McGraw and Arnold’s Atlas of Muscle and Myocutaneous Flaps in 1980.[48]
As is true with a great many revolutionary advances, there is often a simultaneity leading to wider dissemination of the basic concepts. This was certainly true in the case of the advent of myocutaneous flap concepts in promoting the better understanding of the importance of these flaps for a host of reconstructive problems. Two important articles were published in the same year in PRS; the first was by Mathes, Vasconez, and Jurkiewicz, titled “Extensions and Further Application of Muscle Flap Transposition,” in 1977.[49] A little later, in the same volume, McCraw, Dibble, and Carraway published “Clinical Definition of Independent Myocutaneous Vascular Territories.”[50] Mathes andNahai published their book, The Clinical Atlas of Muscle and Musculocutaneous Flaps in 1979.[51]
Within the UM program, there was a sense of being alert to new ideas from national meetings, specialized conferences, personal conversations and bringing them back to Ann Arbor. The importance of this concept was actively supported by both Dr. Dingman and Dr. Grabb. One practical result of this policy was illustrated by the fact that Larry Berkowitz, a junior resident in 1977, went to the first myocutaneous flap dissection course in Norfolk, Virginia, put on by Dr. McCraw. Larry came back and discussed these new concepts with Paul Izenberg, then his senior resident. They discussed these exciting ideas with Dr. Grabb who enthusiastically encouraged them to clearly define the detailed vascular anatomy of all these flaps. Dr. Paul Izenberg recalled, “Little did we know how much this would change the face of all reconstructive surgery. So off we trundled one evening to the cadaver lab and obtained two to three full fresh cadavers. We outlined and prosected ten to fifteen flaps Larry had seen at the meeting including vessels, landmarks, and surrounding anatomy. The entire plastic surgery section showed up a day later for a teaching section. The fun part was that over the remainder of our residency, the residents became the experts on these flaps, helping each other and changing the face of pressure sore surgery and defect closure.”[52] These fresh cadaver dissections were an important advance in the study of surgical anatomy. As mentioned before, no longer were we restricted to the use of embalmed (preserved) cadaver specimens. The availability of these unembalmed anatomic specimens allowed much more precise and detailed dissections especially of small muscles, nerves, and blood vessels. Paul and Larry ultimately defined the anatomy of all the flaps that subsequently entered common usage for a wide variety of reconstructive procedures, including trauma, decubitus ulcers, and reconstruction after cancer resections. These musculocutaneous flaps studied at the time included the gracilis, the tensor fascia lata (TFL), latissimus dorsi, the pectoralis major, the gluteus maximus, the vastus lateralis, and the gastrocnemius. This endeavor led to a much better understanding of differences in patterns in vascular anatomy, which ultimately led to a use of free flaps using direct microvascular transfer. Paul emphasized, “The Section of Plastic Surgery had a great relationship with the Anatomy Department and more importantly, there was no issue getting fresh cadavers for prosection for teaching or individual preparation for surgery. The deaner Bob always had things ready.”[53] We were indebted to the UM gross anatomy department for their willing cooperation in providing the anatomic specimens for our plastic surgery education program through their highly respected anatomic donation program.
Treatment of Decubitus Ulcers
Dr. Paul Izenberg recalled that the availability and reliability of the myocutaneous flap changed the residents’ attitude toward the surgical treatment of these challenging problems. It turned the treatment of these pressure sores into a fascinating exercise to define the best flap and an enjoyable exercise in anatomical dissections to achieve a successful result. Paul recounted, “The surgery on pressure sores which as random or delayed flaps was always a chore. We used to say with the random flaps the weakest part of the flap, the tip, was the most crucial part of the flap, this failure to get a healed wound was not rare. But then it became ‘fun’ for us making it an anatomical challenge with a much better chance of the flap surviving exactly where we needed it! That was a real change from what had gone before.”[54]
It was emphasized to all of us that the most important aspect of preventing these problems in spinal cord–injured patients was the physical therapy teaching about the physical and social changes needed to avoid the pressure injury in the first place as well as avoiding postoperative recurrences.
In the plastic surgery 1972–73 annual report, it is noted that a research project was set up to be directed by Dr. Jim Norris (resident, 1973–74) to evaluate the results of surgical treatment of patients at UM from 1961 to 1971.[55] The objective was to study the rate and reasons for recurrence after surgery, estimate the cost of treatment, and attempt to ascertain the best nursing and surgical care for these patients. In addition, it was decided as a policy to limit decubitus ulcer patients to four on the plastic surgery service at any one time. Dr. Norris (in a letter to me) said, in effect,
The study was intended as an accurate record of all pressure sore cases to determine what factors contributed to complications to apply for a study to investigate the feasibility of a spinal cord center in the state of Michigan. Dr. Dingman was most supportive, but even so we got no response from the numbers of organizations that were contacted. Dr. Dingman was deeply concerned about the treatment of all illnesses. When I told him that I would like to do more for the spinal cord injury patients, he gave me his full support. I have sent to you a paper that was presented at the Annual Residents Conference in 1974. It is entitled, “Pressure sores there must be a better way.” This paper was never published but it demonstrates the kind of support Dr. Dingman gave me. We felt that the treatment of patients with spinal cord injuries demanded special facilities and felt that the University of Michigan Medical Center should have a spinal cord injury center. Dr. Dingman secured probably the second air fluidized bed produced. We set up a small unit and we wrote letters to a number of foundations asking for support for a spinal cord unit. Incidentally, we had the full support of the administration, the neurosurgical service, and rehabilitation medicine service.[56]
It is interesting to peruse Dr. Dingman’s thoughts on the overall management of these difficult patients in the era 1973–74 in the section’s annual report from that year:
We are continuing the study of the etiology and the treatment of pressure sores. Various modalities of treatment such as [random] flaps are being evaluated for their efficacy and complications. Further, various types of pressure relieving beds such as air-fluidized beds as well as the water mattress are being evaluated. These patients continue to present an ongoing serious problem and are in great need of multispecialty care. Our department continues to feel that a cord-injury center would be the most efficient way of utilizing available funds. The average hospitalization of these patients continues to be extensive and the surgical therapy of these sores is one small portion of these cases. Rehabilitation remains the paramount problem and prevention is unquestionably the best mode of therapy for pressure sores.[57]
Fortunately, the advent of seat “cushion” technology and pressure relief with flotation and airbeds became available—their near universal usage has dramatically helped reduce the incidence of these lesions while also avoiding postoperative recurrence.
Clinical Microvascular Surgery
John Markley, our “go-to guy” in this area, reports on the development of clinical microsurgery in Ann Arbor:
In 1970, during my Stanford residency six-month lab rotation, I taught myself microvascular technique in the empty but still equipped micro lab Harry Buncke left behind when he left Stanford. I visited him a couple times at his lab in Mountain View, and used some written material he had generated, and just taught myself, doing rat kidney transplants, end-end arterial, venous, and ureteral anastomoses. This led to doing a rat renal transplant immunologic study with the transplant surgeon at Stanford (Lucas) which was published in 1970. In 1974, I returned to Ann Arbor, and used ENT monocular scopes for some nerve repairs at UM and SJMH. In February 1976, I performed the first digital replant in the state of Michigan, at SJMH, using monocular ENT scope, for a 12-year-old girl’s thumb, which was successful. Several months later, I performed the first digital replant at UM, using monocular ENT scope, on a two-year-old’s thumb (!), which was successful too. This publicity led to more patients in the hand clinic, 1977–78. Although there was by now a binocular scope in the Sargent Lab in Kresge, there was still no binocular scope in the UM ORs. For the next few replants, I had to roll the lab scope through several buildings, two elevators, to either Mott or Main OR to do the emergency cases, often alone in the middle of the night. The vibration from this started to damage the scope and I threatened to stop doing replants unless the hospital bought at least one for an OR. Dr. Turcotte came through with one for Mott, and eventually for the main OR, in late 1978 or early 1979. Replants done in 1976–80 were seventeen digits (sixteen patients) replanted or revascularized by me with resident assistance, one failure, and 94% success. I did some more in 1981–83 also, but don’t have the records for some reason.
From 1978–83, there were elective microsurgical tissue transfers, toe to hand, fibular bone grafts, quite a few free flaps and then micronerve and microvascular muscle flaps for facial paralysis—in addition to the replants, I did elective microsurgical reconstruction during this period at the university, including several toe-to-hand transfers, one or two free microvascular fibular bone grafts, and quite a few free flaps of various sorts. During the same period (1978–83), I did fifteen clinical human facial paralysis reconstructions with various combinations of free graft, transposed muscle flap, and microvascular free muscle flap, all re-innervated by cross-face facial nerve grafts. I had the confidence to do this based on prior clinical work done by others and on the Faulkner/Markley experience, and indeed was rewarded with reasonable clinical improvement in most of these patients (see pp. 40–4—most of the UM plastic surgery residents in this time period were involved in these cases).
Dr. Markley continued, “In 1983, I got together with Dr. Dingman, Jerry Turcotte, Chairman of Surgery, Robert Bartlett, General Surgery, and Dr. William Smith, the head of Orthopedics, to organize a formal replant service for the University of Michigan. Tom Stevenson who had just joined Plastic Surgery, being appointed by Dr. Grabb before he died, became the director of this joint activity between Plastic Surgery, Orthopedic Surgery, and General Surgery.”[58]
For the residents, the combination of Dr. Markley’s superb microvascular training, the invaluable experience they gained in Dr. Acland’s training program in Louisville (see p. 73), and the personal practice time that they had the opportunity for in the Sargent laboratory provided them with excellent preparation to respond to emergency demands such as major extremity traumatic amputations. Dr. Ernie Manders (1979–81) recounted,
We became fine surgeons fast—because we had to BE the surgeon. One example comes to mind to provide contrast for today’s residency. I was on call and a child came in with his foot cut off at the ankle. There was just the most tenuous of skin bridges left, with all major structures divided. I called up fellow resident Mike Watanabe and asked if he would like to help me replant the foot. Sure he said. So we called our staff Lou Argenta and told him what we planned. “Call me if you need me,” he replied. We succeeded and the next few days were a whirlwind of publicity. The child’s picture was on the front page of the Ann Arbor News and Mike and I were called by a local radio station for comments on air. We had our fifteen minutes of fame.[59]
Dr. Bob Gilman (1980–82) recently brought to our attention another dramatic case, the details of which follow:
In February of 1982, the first complete replantation of an amputated hand was accomplished at the UM on the plastic surgery service. A twenty-three-year-old male had sustained a complete amputation of his left hand just above the wrist in an industrial saw accident. The patient had been transferred to the UM from Monroe, Michigan, after he had received emergency care. In the OR, Dr. Gilman and Dr. Gordon Derman, the senior and junior residents on call, dissected the ends of the blood vessels, nerves, and tendons from the cut end of the amputated hand. At the same time, Dr. Louis Argenta, then a faculty staff member, and Dr. Malcolm Marks, another senior resident, dissected the corresponding structures on the cut end of the stump of the patient’s arm. An orthopedic team plated the radius and ulna after slightly shortening the bones to reduce tension on the subsequent vascular repairs. Then the four-man plastic surgery team repaired an artery and two veins. Once circulation to the hand had been successfully established, the second artery and the nerves and tendons were repaired. The patient’s hand survived, and gained protective sensation and enough motion for use as an assisting hand.[60]
As Dr. Izenberg added,
Cases like these partial and complete replantations as well as reconstructions with microvascular tissue transfer have literally become routine since those early beginnings.[61]
Shotgun Injuries to the Face
This was one horrifying and extremely difficult group of patients. The vast majority represented unsuccessful suicide attempts, in which the initial air release from the barrel of the gun in the mouth kicks forward and the main injury is to the midface and jaws. So the patients not only had acute massive wounds but they were also in need of both prolonged reconstruction and psychiatric care. Prior to the era of myocutaneous flaps and free tissue transfer, the reconstructive options were often carried out over several years. These patients’ names remained imprinted in the residents’ memories, and often, as they gathered at meetings, they would say to each other “. . . do you remember Harvey so and so . . .” and recall how one may have done the initial debridement, another a tube flap transfer, and yet a third a bone graft. Reconstructive tools such as a forehead or deltopectoral flap were often inadequate to compensate for the three-dimensional aspects of these complex and massive wounds. The patients were often left having to wear a mask and carry a towel at all times. It was not until the early 1980s that the new reconstructive advances allowed reasonable reconstruction for these desperate patients. As Dr. Izenberg shared, “Still the results with so much tissue missing was always limiting. Hopefully preventive psychiatric care will reduce these horrendous injuries. Face transplant surgery, as it evolves, may also provide more comprehensive reconstruction possibilities.”[62]
Gender Reassignment Surgery
Sometime in the late 1960s, a conference was organized at the request of a UM staff psychiatrist to discuss treatment of transgender patients who had sought care at the university hospital. It was pointed out to us that understanding of transsexualism had progressed to its being considered a specific disease entity and a well-defined diagnosis in the psychiatric literature. The psychiatrist explained that it was a unique condition and not a variant of homosexuality. We were also told that there was no effective or perhaps even justified medical or psychiatric treatment. The current thinking was that, in selected patients, hormonal management and gender reassignment surgery was the best option. Consequently, a team was set up comprising specialists from relevant disciplines, including psychiatry (Dr. Joseph Pearson), psychology testing (Dr. Richard Hertel, PhD), social work, urology (Dr. Joseph Cerny), gynecology/endocrine (Drs. Bob Jaffee and George Morley), and plastic surgery (Drs. Grabb and myself). All potential patients were evaluated by psychiatry, underwent psychological testing, and had a social work evaluation. Additional requirements included cross-dressing as the sex they wished to be, appropriate hormonal therapy, and having lived as a transsexual for up to two years. If successful in these endeavors, then the patients were reevaluated and were required to be accepted as surgical candidates by the entire team. One technique I employed in my evaluation was to see the patient with a medical student who would be unaware of the patient’s condition. If the patient was convincing in manner and appearance to the student, this was an important factor in my final approval. The vast majority of our patients were males wanting to be females and had insurance coverage through the Detroit auto companies where they worked. In general, following surgery, they were usually quite satisfied with their conversion. At the time, we were lining the neo-vagina with skin grafts and contractures in these reconstructions created need for prolonged follow-up for the gynecologists who were the primary caregivers in the follow-up period. This situation eventually led to termination of the program. The female to male patients were more difficult, at that time, as we were limited for penile reconstruction to using delayed, multistaged abdominal tube flaps with an internal lumen for the urethra. The results were less than ideal and tedious for both the patient and surgeon. Even with the limitations and complications, the majority of these transsexual patients were grateful for the team’s sympathetic understanding of their condition and our willingness to be involved in their care. For a variety of reasons, the program was not ultimately sustainable. It was certainly a unique educational experience for us to have a better understanding of this newly described and, in general, poorly understood condition. Newer techniques and more psychological understanding have helped these programs to flourish again within the UM plastic surgery section under Dr. William Kuzon.
Clinical Skin Expansion
Dr. Eric Austad had the most intimate recollections of the program from its inception at the UM:
In one of our occasional conversations over his later years, Radovan recalled to me his initial conversation with implant manufacturer Rudy Schulte, in which he outlined his concept of a tissue expander while driving Schulte to a local airport in the fall of 1975. Driving guests was a universal resident function in the 1970s. Schulte was impressed, and a few months later Radovan received his first expander. It was a two-tubed balloon which allowed inflation or deflation as desired, and his patient presented a particularly difficult problem: a large diabetic foot ulcer which he successfully treated with an expanded cross-leg flap at a local Veterans’ Hospital. As a failure of this rather heroic first procedure would have been a significant setback, Radovan’s confidence and/or luck was remarkable.
In October of 1976, our Section Chairman, Bill Grabb, attended the American Society of Plastic and Reconstructive Surgery (ASPRS) meeting in Boston and returned with the news that a California plastic surgeon had presented a “poster session” describing his work with early versions of what became known as the “Radovan Expander.” This was my first knowledge of Radovan’s work and I wrote him a letter on the university letterhead inviting some interplay of our mutual interests, but got no response. In the meantime, guinea pigs became our basic model for animal work with self-inflating expanders, as no one had actually studied or reported any information regarding the basic safety of the expansion process. Specifically, was this a safe procedure? What cellular changes occurred? Was vascularity compromised? What happened to hair follicles? Was mitosis stimulated or was “malignant transformation” induced? What happened to nerves? In general, did expansion result in useful and safe tissue? We believed, in contrast to Radovan, that these questions had to be addressed before we could consider human trials. In summary, we went first to the laboratory, while Radovan went first to his local V.A.
By the early 1980s, Radovan’s implant became available on a limited basis and other Michigan residents became very involved in the clinical use of expanders. Lou Argenta gained extensive experience with the Radovan expanders, particularly in the head, neck, and breast, and he went on to become a world-class authority on expansion. Ernie Manders presented outstanding results using expanders in nasal reconstruction, electrifying the attendees of the first national tissue expansion symposium sponsored by our section and the Educational Foundation of the ASPRS in May of 1981. In his demonstration of the intricate folds involved, he used his necktie unforgettably. He also went on to become internationally known for his work with expansion and developed and marketed the “croissant expander” and the “differential expander,” which are particularly useful for breast and scalp reconstruction. I chaired that meeting, where Drs. George Cherry, Krystyna Pasyk, Malcolm Marks, and Ken McClatchey all presented both laboratory and clinical work, and both Dr. Radovan and Dr. Gordon Sasaki reviewed their clinical experience. (Dr. Sasaki later wrote a classic textbook on expansion and lectured on its use internationally.) Lou Argenta and I were invited to write the chapter on expansion for McCarthy’s Plastic Surgery, the eight-volume successor to Converse’s book. In February of 1984, Rod Rohrich organized the first international tissue expansion symposium at Oxford, and invited virtually all of the major workers in the field to a most-successful two-day event. Many subsequent courses and symposia followed.
As a final note, I would express my gratitude to Dr. William C. Grabb, my mentor and friend, my fellow residents and staff, and the colleagues worldwide who have helped, challenged, and encouraged my work. If I had not had the privilege of working in Dr. G. Barry Pierce’s lab as an undergraduate, where I first saw and used semi-permeable membranes, and if I never had the experience of observing the sequelae of massive weight loss as a resident at Michigan, I would have missed this entire adventure.[63]
In 1984, along with Drs. Rohrich and Izenberg, I published a case demonstrating the usefulness of skin expansion during reconstruction of the upper two-thirds of the external ear.[64] The expansion facilitated draping of the otherwise insufficient superior retro-auricular skin over the autogenous costal cartilage framework of the reconstruction. This is a good example of one of the many types of deformities where expansion was being use for distinct advantage.
Breast Surgery
1. Reduction Mammoplasty
Reduction mammoplasty was a common operation early in the program. The operation described by Strombeck[65] was the procedure of choice. The technique consisted of the Wise pattern reduction with a horizontal pedicle containing the nipple-areolar complex on the retained breast tissue. As we gained more experience, particularly in reduction of larger breasts, the ease and safety in repositioning of the nipple-areolar complex seemed unreliable. Ron Wexler learned about the McKissock vertical pedicle technique[66] at the 1971 Montreal ASPRS meeting. We discussed it and together did the first case in Michigan and were impressed that the vertical mobility of the pedicle seemed to overcome some of the disadvantages of the Strombeck technique. However, the narrowness of the pedicle and the question of reliability of the vascularity and sensation to the nipple-areolar complex in larger and more ptotic breasts concerned us. When Drs. Courtiss and Goldwyn described the inferior pedicle technique in 1977,[67] it seemed the perfect answer for the types of breasts that might require maximum vertical repositioning of the nipple-areolar complex. Gaining experience in this technique confirmed our initial enthusiasm. In more pendulous and larger breasts, necessitating longer pedicles, we learned the vascularity of the pedicle could be enhanced by designing the base of the pedicle along the entire width of the inframammary fold. Because of the flexibility of this procedure for any size and shape of breast, the mobility of the pedicle, and a greater sense of vascular security, the inferior pedicle technique became the standard procedure for almost all of our reductions. An early exception to the use of inferior-pedicled reductions were high-risk patients with large breasts in whom we wanted to avoid any significant blood loss and who seemed to be well suited for the amputation-free nipple technique. The not-so-infrequent hypertrophic scars in the lateral extent of the inframammary scar were the main disadvantage of reductions using the Wise pattern, particularly in younger patients. Ultimately, this led to a search for procedures with a shorter horizontal scar, which have become more common.
In planning for reductions and ptosis correction, there was always discussion about the proper positioning of the new nipple during preoperative measurements. In an effort to establish better criteria for the ideal nipple placement relative to breast size, together with Mr. Denis Lee of medical sculpture and Art Rathjen from the Dow Corning Company, we proposed a clinical study to clarify this issue. Art was interested in getting accurate measurements of normal, attractive breasts of various sizes to help with Dow Corning’s design for external prosthesis, while Denis was an artist and sculptor, knowledgeable about artistic proportions. Because of my interest in surgical solutions, the most significant measurement for me was the ideal distance from the sternal notch to the nipple in the postoperative breast. We recruited a group of young, female university students who felt they had “normal” breasts and volunteered for the study and underwent measurements and photographs of their breast size and position. The subjects’ breast size varied in bra size from 32 to 38 and cup sizes from B to D. The study documented that the nipple distance was longer with progressive increase in bra size and also increased by cup size. We arbitrarily chose C-cup breast size, which we felt was a reasonable postoperative endpoint for most reduction patients. We discovered that for bra size C cup, the distance averaged almost 21 cm for 32C, 22.5 cm for 34C, almost 24 cm for 36C, and slightly over 25 cm for 38C. This concept was supported by the update Courtiss and Goldwyn reported in 1980 where they also discussed concern about placing the nipple-areolar complex too high.[68] So the usual recommended 22 cm preoperative measurement was too short for many patients. In addition, we learned to take into account the individual patient’s breast skin elasticity and the postoperative effect on planned nipple-areolar position. I am convinced that this study helped us to plan breast reduction so that the nipple-areolar complex was not too high, which, should it occur, was almost impossible to satisfactorily correct. This was also helpful in preoperative planning for breast ptosis patients. A breakthrough in breast ptosis correction was provided by Dr. Daniel Marchac, from France, when he demonstrated the importance in reshaping the breast by splitting the lower pole of the gland up to the areola, and then overlapping the two segments horizontally so that the final improvement in shape and position was not just dependent on the skin reshaping but could be achieved by restructuring the breast tissue.[69]
2. Breast Augmentation
The first Dow Corning gel implants, which contained viscous silicone gel of various viscosities within a silicone (Silastic) envelope, became available in 1964 (after a report by Cronin and Gerow from the University of Texas[70]). Many of the previous nonautologous materials used in attempted augmentations had resulted in serious complications and required removal. This included injected free silicone, much of which was not always of medical grade leading to serious complications. The new silicone implants were thick walled and contained quite viscous gel. They were designed with Dacron patches on the back to stimulate fixation. Originally, they were placed in a subglandular position through inframammary incisions. As an alternative to using absorbable dermal sutures in closing these incisions, which often resulted in suture reactions and delayed wound healing, we introduced a double layer removable subcuticular permanent nylon suture closure, running both in the deep dermal layer and the epidermal layer. This technique insured much reduced possibility of suture reactions and significantly lessened concern of possible extrusion of the implant. Saline-filled implants were also available but were of somewhat limited popularity because both patients and surgeons feared deflation. This potential was greatly reduced by the posterior flap valve being replaced by an anterior-placed injection valve. Early replacement with a new implant after deflation was important to avoid contracture of the existing implant pocket. Spontaneous deflation was actually quite uncommon, and considering the worry arising later regarding the safety of silicone gel, it would have been wiser if we had used them more frequently. Capsular contractures were common with the gel implants (somewhat less with saline filled) and manual “cracking” (putting external pressure of the breast to force the capsular tissue to rupture and allow the implant to relax) became a common office procedure. In the early years following the introduction of gel implants, one manufacturer tried to alleviate the unnatural feel by making a thinner shell and a more liquid gel. As a result, with closed capsulotomy, there was a possibility of extrusion of gel into the adjacent tissues or into an axillary lymph node, a case of which I reported in PRS in 1979.[71] Gel bleed through the implant shell also became more of a problem.
One of the early clinical research projects we carried out in the late 1960s was a psychological evaluation of preoperative augmentation patients in conjunction with two psychiatric colleagues. The work was not published but the analysis of over thirty female patients in their third decade revealed that the vast majority seeking augmentation did not manifest any serious psychological problems. We also learned that by using open-ended questions in patient interviews, we gained a lot of information about the patients’ deeper feelings about themselves that allowed us to be more understanding in our care of them.
We quickly realized that with augmentation mammoplasty, intraoperative implant selection was impractical. We needed a method whereby we could obtain patient approval of the appropriate implant size preoperatively. The simplest solution we found was for the patient to buy a bra with the proper number for her chest size and the new cup size she wished to be. In the old Jacobson’s retail store across the street from our downtown Ann Arbor office in the Tower Plaza, I identified a helpful salesperson who volunteered to advise these patients after I described what we were trying to accomplish and helped them select the appropriate size bra under various styles of clothing. The patient would then bring this new bra to a second office visit. Using available implant sizers in the bra allowed the proper size implant to be selected. Involving the patient in the size selection led to a much greater degree of postoperative patient satisfaction.
Although capsular contractures were not as great a problem with saline implants,we began to try to alter the configuration of the scar tissue contracture by the use of soluble steroids placed in the implant. Dr. Louis Argenta and I reported long-term results in a series of patients with a fairly high dose of the soluble steroids within the implants.[72] The surrounding tissue atrophy leading to implant ptosis in the patients was common but inconsistent. We did not feel that the dosage would necessarily be the only factor. We recommended that even if smaller doses of steroid were used in any prospective research project with saline-filled implants, there should be long-term follow-up. Some of these adverse effects did not appear for several months or even for more than a year after the implant was placed.[73] With a lower dose, it might be an even slower process. Fortunately, in this group of patients, atrophic changes that precipitated the ptosis were reversible following removal of the steroid containing implant. If the implant were replaced with one not containing steroids, we found that we could make a new pocket in the correct position behind the existing one that would lead to a satisfactory result.
Placement of the breast implants under the pectoral muscle had first been described in 1968. The report of one hundred cases by Kenneth Pickrell et al. in 1977[74] as well as presentations at national meetings by Paule Regnault, a Canadian plastic surgeon from Montreal, renewed our interest in that technique. Because of the resulting much lower incidence of capsular contracture, the subpectoral placement became our standard approach. Dr. Thomas Hudak (resident, 1968–70) was our visiting professor in 1980 and shared with all of us his preference and experience with the procedure. He recollected, “I enjoyed my time as the Visiting Clinical Professor (1980) when I taught the residents the technique for submuscular breast implantation, a procedure that when properly done significantly decreased capsule formation. The implants for augmentation were placed submuscular with partial release of the medial attachment of the pectoral muscle. The release of the muscle prevented the implants from being pushed laterally from beneath the muscle.”[75]
Once we realized that the normal relationship of the pectoral muscle covered only the upper half of the implant and that the lower part could be placed in an expanded subglandular pocket contiguous with the subpectoral position, we were able to use larger implants that gave a more satisfactory and natural result. The peri-areolar incision, although popular with many, was not, for some of us, convenient in dissecting the subpectoral space. In the case of unmarried women, the transaxillary approach for subpectoral breast augmentation became popular to avoid any chest wall scar. In the mid-1970s, a special subpectoral dissection instrument was designed by Drs. Dingman, Agris, and Wilensky.[76] The latter two were both residents in 1976.
During these years, we enjoyed many advantages of the free exchange of information among plastic surgeons from regional hospitals, such as Providence and Beaumont. New techniques we used included details about transaxillary approach using inflatable implants and the marked advantage of intercostal Marcaine blocks to supplement local anesthesia with sedation. Placing the blocks in the mid-axillary line with small, short needles avoided the incidence of pneumothorax. This combination worked very nicely in outpatient augmentation procedures in an outpatient or office OR setting.
3. Prophylactic Mastectomy
In the mid-1970s, there was a renewed concern about the possibility of progression of premalignant breast disease in patients with a maternal history of breast cancer. At SJMH, we formed an interdisciplinary team of general and plastic surgeons, radiologists, and pathologists who met, evaluated the patient, and recommended appropriate treatment. This was at a time before there was knowledge of a high-risk breast cancer gene. There was an ongoing argument about the rationale for prophylactic mastectomy that left a minimal amount of subareolar breast tissue in place even with coring out the nipple versus complete mastectomy. Our impression, at the time, was that the former procedure gave patients considered high risk, and particularly those who had lost family members to breast cancer, considerable peace of mind. Obviously, they required close follow-up by a breast oncologist. To my knowledge, there was no breast cancer that showed up later in any of our patients.
In these prophylactic mastectomy patients, there was always concern about viability of the skin flaps if adequate breast tissue removal was accomplished. The use of low-dosage and slowly injected intravenous fluorescein evaluation proved to be very helpful in determining the viability of the skin flaps and allowing a single-stage reconstruction when indicated (see p. 77).
In 1978, Jarrett et al.[77] reported improved reconstructive results by placing the implant not only subpectoral but also under adjacent portions of the serratus anterior muscle, to create a total submuscular position. This idea was rapidly taken up at Michigan and became the method of choice in this group of patients. In all categories of breast reconstructive patients, complications of implant extrusion and capsular contracture were greatly reduced by placing the implants totally submuscularly, and appearance and patient satisfaction were greatly improved. Another option for autologous reconstruction without implants, particularly in complicated secondary cases, was the transverse rectus abdominis myocutaneous (TRAM) flap (for discussion, see “Breast Reconstruction” section). This could be performed as a bilateral procedure in patients with bilateral prophylactic mastectomies who had unsatisfactory implant reconstruction or for any patients requiring removal of bilateral damaged implants.
4. Breast Reconstruction
Early in the program, breast reconstruction did not receive much attention from general surgeons, and there were not many patients requesting it. Dr. Paul Izenberg recounted, “We began with some very basic approaches placing silicone implants under the mastectomy flaps resulting more frequently than not in severe capsular contracture looking like a baseball, being painful, no sense of symmetry, and occasional extrusion. But many of these patients kept the implants as it meant an attempt to return to ‘normal.’”[78] The deformity from radical mastectomy, with an absent pectoralis major muscle and often a chest partially covered by a split-thickness skin graft (STSG), was a formidable challenge for reconstructive surgeons. Dr. Izenberg continued, “There was very little that could be done with the radical mastectomy patients at this time or those with severe radiation damage, not an infrequent sequela of the treatment.”[79] With the few of these patients on whom we attempted reconstruction, we used either large rotated, delayed flaps or staged abdominal tube flaps, but the results were not satisfactory. As Dr. Izenberg said, “They didn’t look good, took a very long time, and there was lots of patient morbidity.”[80] When myocutaneous flaps became available, a better option was to transfer the latissimus dorsi myocutaneous flap with a skin island to the anterior chest to provide muscle and skin cover for an implant. Better results were obtained, but not all patients would accept the donor site. Dr. Izenberg observed, “Once modified radical mastectomies became the standard of care, we learned to place the implants under the [preserved] pectorals muscle with the lower one-third covered by external oblique fascia and rectus fascia. Not perfect but better. As mentioned previously, tissue expansion came along with introduction of the Radovan expander [constructed with a separate valve attached with a tube] which caught on quickly and we began using it, elevating some lateral serratus in addition to the pectoralis muscle to help with cover.”[81] This was another significant improvement but still required the use of an implant with the potential associated problems with capsular contracture.
In 1982, Paul Izenberg and I became aware of the TRAM flap, which was originally labeled a transverse island flap, but was really the transverse rectus abdominis musculocutaneous flap, first reported by Hartrampf, Sheflan, and Black in 1982.[82] As we were learning the technique, Paul and I did the first few cases as cosurgeons. We both felt the collaboration was well worthwhile, as together we were able to more efficiently work out many of the technical details of the new and exciting reconstructive procedure. This ushered in a whole new era of breast reconstruction using only autogenous tissue, particularly well suited for breast cancer patients having undergone radiation. With more experience, it became possible to use this as a means of primary reconstruction at the same time as the mastectomy. An added benefit was that the patient also received an abdominoplasty in the process. In 1984, Dr. Roger Friedman, as a senior resident, along with his faculty mentor Dr. Lou Argenta, did one of the first microvascular transfers of a TRAM flap for breast reconstruction, combining the microvascular techniques described by Dr. Markley and knowledge of this new flap design.[83]
Nipple reconstruction became available and could be accomplished in selected cases by sharing a large contralateral nipple-areolar complex.[84] In most cases, however, a small local skin flap to create a nipple combined with tattooing to create the areola was very successful.
During this time, a significant number of patients and providers joined a movement to force insurance companies to cover postmastectomy reconstruction. I had a patient who, with my help, lobbied the Ohio state legislature to mandate insurance coverage. Ohio’s passage of this mandate was a step toward subsequent national acceptance for insurance coverage for breast reconstruction and, eventually, for appropriate surgery of the opposite breast by all health insurance companies including Medicare and Medicaid.
Cleft Lip and Palate
In any discussion on this subject, we must emphasize Bill Grabb’s interest and devotion to the field of cleft lip and palate. This is well illustrated by his very comprehensive book, Cleft Lip and Palate, published in 1971 with coauthors Sheldon Rosenstein, DDS, professor of orthodontics at Northwestern Dental School, and Kenneth Bzoch, PhD, at the University of Florida.[85] It covered all aspects of the cleft sequence, was very authoritative for its time, and received good reviews. In addition to Dr. Grabb, UM contributors included Dr. Dingman along with Ted Dodenhoff, a resident at the time, with a chapter on surgical correction of mandibular deformities in mature cleft patients. The book also included my contribution of a chapter on oronasal fistulas.
As we mentioned in chapter one, Dr. John Kemper was an early practitioner in the treatment of clefts and beautifully stated the case for the multidisciplinary approach to these patients: “Rehabilitation of the cleft palate patient may be likened to a great drama in which there are a number of actors, each playing an important role under the direction of one individual, the surgeon, who is responsible for its management and production. The most distinguished and important character, the central figure in the play, is the patient, whose successful performance is dependent on the skillful guidance of the director [the surgeon] and the combined efforts of all other members of the cast.” In this presentation, he went on to discuss the other critical aspects of care of the cleft patient, including feeding, reassuring the parents that the child will in all probability develop to be a completely normal child, and emphasizing the importance of their continued cooperation in the rehabilitation of the child over a number of years. He also acknowledged the importance of specialized surgical training and achieving not only technical skill but also good surgical judgment. He also stressed the importance of early repair of any lip defect to help mold the displaced alveolar segments, the careful observation for ear infections, and the postsurgical consultation with speech therapists, as well as early institution of active and passive palatal exercises. He closed by stressing that dental care be carried out at the right time and in the right manner including appropriate consultations with a prosthodontist. Finally, he stated the responsibility of the surgeon for timely correction of the commonly associated secondary facial deformities.[86] All of these important aspects taught to us by Drs. Dingman and Grabb formed the basis for our care of the various aspects of the oral cleft deformity. A significant aspect of our care for these children was the financial support available through the generous and well-administered Michigan Crippled Children’s fund. This provided total financial support for the comprehensive care of cleft lip and palate patients until eighteen years of age.
1. Unilateral Cleft Lip (UCL)
Although there was some pressure by parents and relatives to operate on babies with unilateral clefts as newborns, we felt that it was better to wait until about three months of age. Another rule Dr Grabb insisted upon and we all followed was the 10-10-10 marker: 10 weeks, 10 lb, and 10 gm of hemoglobin. The wait usually assured the baby was healthy but, just as important, that the parents had a chance to bond with the child. We watched the parents go from seeing only the cleft deformity when they looked at their newborn child to seeing, at two to three months of age, a beautiful healthy baby with a limited defect. We came to realize this early bonding of the parents and the cleft baby was essential for the future welfare of the child. Many of our cleft patients were referred from the newborn nursery at SJMH. Our good relationship with the pediatricians facilitated our seeing the patients in the hospital shortly after birth. This allowed us to reassure the family that they could expect that their child would be able to lead a normal life before any discussion of specifics of surgical procedures or speech therapy.
After Dr. Millard published his classic description of the rotation-advancement technique for repair of the UCL in 1964,[87] this became the standard approach that we used at the UM. It worked well for many clefts. However, we began noticing less-than-satisfactory results in creating lip symmetry in the more severe complete unilateral clefts with excessive vertical shortness in the lateral segment. At a UM-sponsored Cleft Lip and Palate conference in the early 1970s, Dr. Ernie Kaplan from Stanford[88] was the first person to propose a modification of the Millard technique for UCL rotation incision when a back cut was required. He proposed making a slightly curved incision directly over to the lower extent of the proposed back cut, thereby avoiding the curved incision extending up to the columellar-labial junction. This concept, together with Dr. Robert Pool’s modification of the rotation-advancement technique, made it applicable to all degrees of the UCL deformity. Dr. Pool, from Beaumont Hospital in Michigan, described this approach to us in Ann Arbor in a series of lectures as visiting professor (Photo 54).[89] The two main aspects of this modification were to have the rotation incision on the cleft side be placed slightly below the critically important aesthetic unit of the labial-columellar junction. This distance would increase proportionally to the degree of shortness of the lip on the cleft side and be limited to a maximum of 4 mm. The other important technical aspect was that the upper level of the lateral segment advancement flap was placed below the alar base to the same extent as the medial segment rotation incision was inferior to the columellar-labial junction. This idea was stimulated by his recognition of the difficulty of rotation advancement in cases where the dimensions of the lateral lip segment are deficient.[90] This design allowed the lateral segment, when advanced, to unfold and its vertical height to lengthen. The technique proved to be very adaptable to all variations of severity of the UCL. Dr. Markley, using his well-developed sense of spatial relations and mathematical skills, helped the rest of us to understand the subtleties of this procedure. After I had experience using it, I became convinced of its amazing versatility in the repair of all varieties of primary unilateral clefts as well as for secondary revisions and was encouraged to continue to use it and also to teach it. I feel very indebted to Dr. Pool for his willingness to share and teach his technique.
2. Bilateral Cleft Lip (BCL)
In those early days, as we were one of the few major cleft referral centers in Michigan, we always seemed to have a greater proportion of the more challenging BCL patients compared with UCL patients. A lot of these patients came from the Upper Peninsula of Michigan. They were often referred directly to Drs. Dingman, Grabb, and me both as newborns and as older children. In regard to surgery for BCL, I had an amazing experience at a national meeting when a “light bulb” went off and suddenly I realized that there was a good solution to a previously intractable problem. We all had appreciated how difficult it was to achieve satisfactory aesthetic and functional repair in cases of complete BCLs. Dr. Ralph Millard, at the ASPRS meeting in 1970, presented his idea for unifying the orbicularis muscle from the lateral segments in the midline behind a narrowed prolabium in the primary repair of a BCL. It was published in 1971.[91] The results shown were so striking that there was instantaneous recognition in the audience of how important this modification was. Soon after that meeting, I saw a teenage patient with a typical secondary BCL deformity with orbicularis muscle bulging in the lateral segments and deficient prolabial vermilion. The result of my revising the lip by bringing the muscle together in the midline and using the excess lateral vermillion to replace the previously inadequate and mismatched prolabial vermilion was dramatic. I presented the case at ASPRS, and it was then published in PRS in 1974, coauthored by Don Greer and Gary Nobel who were both residents at the time.[92] After seeing the published article, Dr. Millard asked whether he could include a description of the key points of the case with the photographs in his second volume of Cleft Craft.[93] I felt much honored by that request. Ultimately, muscle union became a standard feature of both primary and secondary BCL repair at the UM. As described in Millard’s 1971 article, we were also banking the “forked flaps” arising from narrowing the lateral edges of the prolabium to later use for lengthening the columella that was almost always too short.
Often, we were confronted with a child with a previously repaired BCL who had a tight and retruded upper lip and a full, redundant lower lip. Shifting a full-thickness Abbe flap on a vascularized vascular pedicle from the lower to the upper lip corrected the imbalance and allowed the insufficient philtral tissue, still attached to the inferior aspect of the columella, to be shifted upward to produce columellar lengthening. An article published in 1971 by Drs. Dingman and Grabb states clearly the difficulty we all came to appreciate about the BCL and palate. As quoted from this article, “Problems of the double cleft are infinitely greater than those exhibited by a single cleft; the difficulties are multiplied several times. The bilateral cleft lip and palate remains one of the most challenging deformities in all of plastic and reconstructive surgery.”[94] The article recommended early release of the tethered columella using Millard’s “forked flaps,” closure of the soft palate between fifteen and twenty-four months, and delaying closure of the hard palate until more complete maxillary growth occurred.
3. Cleft Palate
One of the major early advances in the management of cleft palate patients was recognition of the potential for damage to the middle ear caused by dysfunction of the Eustachian tubes in infants and young children. In collaboration with our ENT colleagues, we instituted prophylactic treatment in all cleft palate patients either when the associated cleft lip was repaired or when an isolated cleft palate was repaired at about one year of age. Myringotomy was performed, fluid removed, and a small silastic tube inserted to allow equalization of pressure in the middle ear through the eardrum, thereby bypassing the dysfunctional Eustachian tube. Avoiding hearing loss was especially important in cleft patients learning speech. With growth and palatal closure, eventually the tube could be discontinued in the older child once normal Eustachian function developed.
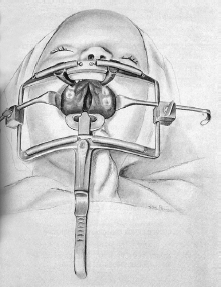
As far as the actual palate repair was concerned, we were forever trying to find the correct balance to achieve acceptable speech and avoid maxillary growth disturbance, and through these years, it was an ongoing process. The operative visualization and the surgical access to these frequently demanding repairs were greatly facilitated by the availability of the famous and widely used Dingman mouth gag (Photo 55).[95] The mouth gag was formulated and manufactured by Richard Sarns of the Sarns Inc. engineering firm in Ann Arbor, Michigan. Mr. Sarns told me that Dr. Dingman had invited him into the OR to visualize the need for improved instrumentation to increase safe cleft palate operative exposure as well as to minimize the necessity for unnecessary assistants around a very small operative field.[96] Sarns designed a multipurpose instrument on a square frame that met four essential requirements: adjustable tongue depressors to stabilize the endotracheal tube as well as the patient’s tongue; provide a secure, as well as anatomically safe, open mouth position; lateral cheek retraction; and tightly coiled small springs attached to the upper and lower horizontal bar to hold multiple suture lengths after they had been tied in place to allow them all to be trimmed in a single maneuver. Three different sizes of tongue depressors were made available to allow the instrument to be used in all ages of cleft patients. This well-designed instrument quickly became an essential mainstay for all types of cleft palate surgery and is now in worldwide use.
The idea of closing the soft palate first, allowing the hard palate cleft to narrow, and delaying hard palate closure until eruption of the primary first molar at around thirteen to nineteen months was very appealing for minimizing potential for palatal narrowing except for the disadvantage of an extra operation. Once we learned that avoiding undermining posterior to the maxillary tuberosity would minimize growth disturbance, we began to rely on the straight line von Langenbeck closure of the entire palate, often reinforced by a vomer flap in wider clefts. Palatal lengthening procedures using setback techniques were also performed but had the disadvantage of leaving too much raw surface in the anterior palate. Dr. James Hayward, DDS, head of oral surgery at UM, taught us that extending closure of the soft palatal layers posterior to the uvula (low-draped palate) did in fact functionally lengthen the palate, and we began to use this technique routinely. Finally, recognition of the abnormal anterior orientation of the levator muscle in the cleft encouraged us to reorient the muscle in a horizontal end-on position in the closure of the soft palate. When Furlow’s double Z-plasty procedure for closure of the soft palate[97] was reported, we began to use it. We felt it was the operation of choice for submucous clefts but could be problematic on quite wide complete clefts.
In response to the early uncertainty about which cleft closure would produce the best overall long-term result, Dr. Grabb proposed a prospective, randomized long term study.
The project was described in the 1972–73 annual report. A cooperative study of cleft palate surgery would begin in 1970 and was planned to run for at least seven years. Its purpose was to objectively determine which of four different cleft palate repair procedures, selected randomly, would give the best results with regard to speech, facial growth, and hearing.[98] The planning included dentistry, speech, and ENT, in addition to plastic surgery. It was hoped that there would be conclusions within ten years. To acquire the ninety patients[99] needed for the study to have the best chance of statistical significance, Dr. Grabb invited seven other plastic surgery programs from the United States and Canada to participate in the study. To my knowledge, this was one of the nation’s first multiinstitutional prospective outcome studies. Unfortunately, when Dr. Grabb died, a lot of the momentum for the study dissipated and the study eventually ebbed before enough patients could have the necessary and required long-term evaluation.
4. Cleft Palate Clinics
One of the most important concepts in the management of cleft lip and palate patients was to incorporate a multidisciplinary approach from the very beginning of their care. From the early days of plastic surgery in Ann Arbor, there was general agreement that this was necessary for the best outcome for patients with these deformities after their lip and/or their palates were repaired. Consequently, before the UM section was formed, there was a cleft lip and palate clinic at SJMH. Among the specialties represented, other than plastic surgery, were pediatrics, ENT, speech therapy, and the dental specialties, including pedodontics, orthodontics, and prosthodontics. Dr. Harlan Bloomer of the speech and language department at UM was a long-standing member. Dr. Gene Buatti, DDS, an orthodontist, was also a loyal participant. The children were all seen after the age of five then, at least once a year by all members of the team. This allowed for close coordination in all decisions about care as the child matured. Clinic visits lasted until final treatment procedures were carried out or when the patient reached maturity in the late teenage years. Occasionally, the clinic would see older children or even adults who still needed treatment or were being seen for the first time. After the Section of Plastic Surgery was formed in 1964, the university hospital cleft clinic included, in addition to the specialties listed above, oral surgery, genetics, and pediatric social workers.
5. Presurgical Palatal Orthopedics
In treating the UCL alveolar deformity, initially we used a preliminary lip adhesion for the very severe complete cleft. This procedure effectively narrowed the alveolar cleft so as to facilitate the definitive lip repair. However, the scarring from the initial adhesion often made the definitive repair more difficult. We began to use Dr. Pool’s suggestion of having the parents, right from the baby’s birth, place Steri-Strip tapes on the lip across the cleft in the lead-up to lip repair. We found that this significantly narrowed the alveolar cleft and accomplished almost as much as the lip adhesion did without the additional operation. There were cases, however, where there was significant collapse of the lateral alveolar segment and we needed some way to expand it. Dr. Latham, DDS, had described preoperative maxillary orthopedics as early as 1976.[100] His expansion device was in two pieces, connected by an adjustable plate, and was pinned to the maxillary segments in an operative procedure. The parents gradually expanded the palate by daily turning a small screw. The construction of this device obviously required the collaboration of a pediatric prosthodontist, and one was not always available. The Latham device became most useful in the management of bilateral clefts for controlling the ideal width of the lateral alveolar segments and to help reduce the abnormal forward positioning of the premaxilla. There was general agreement among us that surgical repositioning of the forward projecting premaxilla was detrimental and that it could lead to midface retrusion. We felt getting the lip closed and orbicularis muscle union behind the narrowed prolabium would allow the premaxilla to end up in a more normal position. With wider complete bilateral clefts, bilateral lip adhesions were frequently required, and if there was a very wide cleft and a very protrusive premaxilla, then we would have to stage the adhesions, one side at a time. There was still a fairly high incidence of adhesion dehiscence. One of the ways we found to reduce that possibility was by taking the tension off the closure by a retention suture placed in the muscle through both sides of the lip right under the alar bases. It was an improvement, but we still had a long way to go in the management of the bilateral cleft.
6. UCL Nose
Sometime after Harold McComb, from Australia, described his ideas of repositioning the nasal alar cartilage on the cleft side in 1976,[101] and we began to use the technique, our results were encouraging. It was very appealing because, in comparison with other described techniques, it could be accomplished through the existing incisions necessary to repair the lip. As McComb had described, we used two permanent suspension sutures through the upper edge of the cleft-side lateral crus passed subcutaneously and brought out at the nasion and tied over a Steri-Strip. Obviously, this required some subcutaneous dissection from the alar cartilage up over the cartilaginous and bony vault. We discovered it was best to slightly overcorrect the inferiorly displaced alar cartilage. We were pleased with the initial results and gratified when Dr. McComb published his ten-year follow-up in 1985.[102] Correcting the position of the cleft-side alar cartilage completely precluded having to do any nasal tip surgery in the preschool age child. As teenagers, the patients often did need a secondary corrective rhinoplasty to achieve better symmetry in the nose, particularly around the tip, but the remaining deformity was much less severe than in patients who had not had primary nasal repair.
7. Nasoendoscopy
In the mid-1970s, when 3-mm flexible fiber optic endoscopes became available, we realized that this would give us the opportunity to directly view the anatomy of palatal closure through the nasopharynx. We had always been able to diagnose velopharyngeal insufficiency by analyzing the patient’s speech, and confirm velopharyngeal incompetence by listening to the air leak during speech with a tube placed between the patient’s nares and our ear. However, an oral view of the palate in action did not give us the specific information we needed to assess not only the adequacy of closure of the repaired palate but also the specific anatomy of it to assist in planning the advisability of nonsurgical therapy or which type of surgical correction would be necessary. The available radiologic studies required x-ray exposure and did not always give us the desired three-dimensional information we needed. We began to use the endoscope in the outpatient clinic on selected older children. Anesthesia was provided by using topical nasal sprays, and we were impressed with the clarity of palatal function on the video as the patient spoke test words and phrases. Being able to assess the movements of the lateral and posterior pharynx was an added benefit. Endoscopy had become an extremely helpful guide in selecting the procedure likely to be most successful for each of these patients. The results were essential in deciding between the more traditional posterior pharyngeal flap, which was basically static, and the innovative dynamic pharyngoplasty, originally described by Orticochea in 1968[103] and modified by Jackson and Silverton in 1977.[104] Gradually, we were able to study younger children, and many of them enjoyed looking at the recorded videos after the exam. From the beginning, we collaborated with the speech therapists who worked with us in the cleft clinic, and ultimately, they took over doing the exam and were more successful than we were in getting younger children to cooperate. The endoscope was a tremendous advance in the follow-up of all our many cleft palate patients. Drs. Izenberg, Rohrich, VanderKolk, and I published our early experiences in 1985.[105]
8. Alveolar Bone Grafts
Over the years, a more collegial relationship developed with the oral surgeons participating in the cleft clinic conferences. We exchanged information freely when we discussed the patients who had been evaluated. One of the benefits of this was that Dr. Ray Fonseca, DDS, head of oral surgery at the time, generously shared with me his technique for closing and bone grafting alveolar clefts in the mixed dentition patient.[106] This involved creating a bed for a cancellous iliac graft by turning in toward the nasal floor the mucosa of the fistula tract, and widely undermining and shifting the palatal tissue to close the nasal side. Undermined maxillary gingival flaps were then shifted toward the midline to close the oral side. This procedure was a big improvement over previous techniques. The iliac bone graft site was rendered much less painful for these children by avoiding any muscle dissection and creating a periosteal-cortical flap for access to the medullary layer that was closed over the extraction site.
Rhinoplasty
Dr. Dingman’s openness to new and better ideas led the rest of us to be aware of new advances in rhinoplasty. His friendship with Dr. Joseph Tamerin from New York City led Dr. Tamerin to be visiting professor at UM in 1969. Dr. Tamerin operated on one of our patients with a resident assisting. He used the intracartilaginous (cartilage splitting) incision for approaching the tip in rhinoplasty. This demonstration and the resulting natural-looking tips in the patients that he presented later that day in conference encouraged all of us to start using that approach, moving away from previous experience with either the intercartilaginous retrograde approach or the delivery technique. Then, in 1971, Dr. Dingman sent Carl Berner, resident 1970–72, to an ENT facial plastic surgery rhinoplasty conference, and Carl brought back all sorts of new concepts, including the open approach, that we discussed and began to use with difficult tips, particularly in teaching situations. Carl reported to me recently, “I remember how thrilled I was to be sent by ROD to ‘spy’ on the facial plastic surgeons regarding their techniques. I had no idea how I would be received, as at that time there was some animosity between the specialties. I need not have been concerned. Dr. Dingman’s reputation preceded me. I was welcomed by all. I believe what I learned was valuable to me in my practice and to all of us. Rhinoplasty was a big part of my practice. What I learned through the rhinoplasty meeting gave me more confidence.”[107] The friendliness and respect shared by Dr. Dingman and those ENT facial plastic surgeons was in part due to the respect Dr. Dingman had gained by his participating in their teaching conferences and having accepted ENT trainees into our residency program. He once said to me, “There should be no limits on education. You should be willing to teach to whomever wants to learn.”[108]
Another “light bulb” experience occurred at an ASPRS meeting in Houston in 1974. Dr. Jack Sheen presented his revolutionary work on the correction of secondary rhinoplasty. We had all been struggling with these deformities. To correct them, we had been removing scar tissue and further reducing dorsal structures to correct the common “pollybeak” deformity. Dr. Sheen turned the tables on us as he showed markedly improved results using augmentation of tip, dorsum, and radix rather than making any further reduction. The underlying concept he presented was to increase the structural support and expand the skin envelope, thereby improving shape and function. I can remember a collective “gasp” in an audience of about one thousand five hundred plastic surgeons as Dr. Sheen presented what I interpreted as amazingly good results. A better understanding of the underlying concepts of that presentation, published in PRS 1975,[109] helped us to define the factors in evaluating a primary rhinoplasty patient that, if uncorrected or made worse, could lead to the necessity for a secondary rhinoplasty. These new concepts helped us to improve the treatment of secondary patients as well as better evaluate our primary rhinoplasty candidates.
Sometime during the early 1980s, I was exposed to the idea of suture techniques to control the shape and position of the tip cartilages. This happened at a university hospital teaching conference in ENT to which I was invited to hear the visiting professor, Dr. Gene Tardy, a well-known facial plastic surgeon. I realized that it was a real advance in rhinoplasty surgery to have available a technique that was very effective, nondestructive, and reversible. Based on this experience, we modified our techniques and significantly improved our results.
Dr. Dingman always placed a high premium on accurate and easily understood terminology to eliminate confusion in discussion of operative details. An example of this attention to detail appeared in the article he and Paul Natvig published in PRS in 1982, near the end of both of their careers, clarifying the incisions for rhinoplasty.[110] This article was an effort to clarify by description and illustrations the details and proper names of the incisions used to gain access to the alar cartilages during rhinoplasty. The important distinction between the oft-confused infracartilaginous incision at the caudal edge of the alar cartilage and cephalic to the soft triangle, and more caudal marginal (or rim) incision was graphically illustrated.
In addition to all this stimulating information, the advent of residents coming through the program with prior ENT training gave all the other residents, and particularly those of us on the faculty who were interested in rhinoplasty, an ongoing tutorial on surgery of the nose, especially with regard to the septum and nasal physiology. This group of residents had been schooled in the technique of open rhinoplasty, which had advantages for correcting complicated aesthetic tip deformities, for secondary cases, and especially for cleft lip nasal deformities. As we began to use the open technique, the obvious advantages for OR teaching became evident. One of the most practical suggestions concerning technique came from Erlan Duus (1983–85). He introduced me to a dental instrument, the amalgam tamper, for ease in defining the proper dissection plane in septal surgery. Those old enough to have had dental fillings might remember seeing one. The exact instrument he recommended has small sharp serrations on the square end. I quickly realized that it was very efficient for “scratching” through the perichondrium of the nasal septum down to the proper layer for dissection without cutting into the cartilage. It seemed to me far superior to either scissors or a knife. I found that, after starting the dissection, if I got out of the proper layer, a few “scratches” would put me back into the proper dissection plane.
Dr. Jack Gunter had been acting head of ENT at University of Texas Southwestern (UTSW), prior to starting his plastic surgery residency with us in 1978. Shortly thereafter, he and I recognized our mutual interest in rhinoplasty. He took the time to discuss with me most of my preoperative rhinoplasty cases. He taught me many of the fine points of clinical analysis and operative technique of rhinoplasty that he had learned both from his ENT training and from his fellowship with Dr. Jack Anderson, a well-respected facial plastic surgeon. After he completed the residency at Michigan in 1980 and went back to Dallas, Jack and I talked several times and came up with the idea of a postgraduate course including cadaver dissections, didactic instruction, and patient presentations. We both felt it was necessary to augment plastic surgery rhinoplasty training for residents, and there was also a need for teaching these basic ideas to recent postgraduates. The first course was at UTSW in Dallas in 1982, where Jack was a faculty member. He recruited other faculty from Dallas and Houston, including Sam Stal, Steve Byrd, and John Tebbets. We talked about having a meeting the next year in Ann Arbor and then alternating sites every other year, but Bill Grabb’s sudden death put that plan on hold. By the time everything settled down at UM, Dr. Rod Rohrich, another one of our alumni (finished in 1985), had graduated and gone to UTSW to join the faculty. Together with Rod and Jack Gunter, the annual Dallas Rhinoplasty Course became well established there and has continued as one of the premier rhinoplasty international conferences for over thirty years. The formal and informal discussions among the faculty, which included some from ENT, were the stimulus that eventually led to the formation of the Rhinoplasty Society. The nasal anatomy video that Paul Izenberg, Bob Gilman (1980–82), and I created for basic teaching in the Dallas course continues to be useful and a valuable part of the curriculum for teaching our residents rhinoplasty at Michigan.
Congenital Protruding Ear Deformity
Several events early on contributed to the evolution of our otoplasty technique for protruding ear deformity. Dr. Stenstrom, in 1963, published his technique for shaping the curve of the antihelix with linear abrasions.[111] Dr. Dingman was impressed that this was an excellent idea, and he then developed a new instrument by taking the two halves of a Brown Adson forceps, bending the stems in two different directions and putting a handle on each one so the instrument in the surgeon’s hand would easily follow the curve of the antihelix of both the right and left ears. This was the origin of the Dingman ear cartilage abrader, another of ROD’s many practical and ingenious modifications. At about the same time, Mr. John Mustarde of Scotland, later to honor UM as visiting professor, described the use of mattress sutures to create a gently curved antihelix.[112] In younger children with more pliable cartilage, probably with either the abrasion or the mattress sutures, the correction would hold up over the long term. However, we felt that it was safer to create the curve by abrasion and then use the mattress sutures for more secure reinforcement. This combination of techniques seemed to be particularly useful in older children or adult patients where more mature, stiffer cartilage might cause the deformity to reoccur after either simple abrasion or just the mattress sutures. Later, in 1979, Dr. Isaac Peled, one of our fellows from Israel, and Dr. Dingman described a modified technique.[113] The final important key to success in our otoplasty evolution was provided by Dr. David Furnas in 1978 whose published article demonstrated that reducing the overprojecting posterior concha wall did not require cartilage excision but it could be repositioned posteriorly with the use of concha-mastoid sutures.[114] The repositioning of the concha was aided by slightly thinning the floor of the concha and removing adjacent soft tissue just posterior to it, thus leaving a space in which the concha could be set back. Another technical aspect was realizing that the standard elliptical postauricular excision when closed could overcorrect the frontal profile of the middle of the external ear. We found that a dumbbell-shaped incision was more desirable and much less likely to cause overcorrection. Otoplasty for protruding ears was one of the most satisfying operations for the patient and surgeon. It was a delight to observe the excited positive response when patients, both young and old, viewed themselves in a mirror at the time of dressing removal.
Face-Lift Experience
The procedure of face-lifting early on was basically a subcutaneous dissection and tightening of the skin, frequently associated with bilateral upper and lower lid blepharoplasty. All this began to change after the publication of Dr. Tord Skoog’s book in 1974 about the deep fascial dissection in the face.[115] Then, in 1976, Drs. Mitz and Peyronie published a detailed description of the SMAS (subcutaneous musculo-aponeurotic system) fascial layer in the face.[116] Because of the improvement in the nasolabial fold and less tension on the dissected skin cheek flaps, there was subsequently a lot of enthusiasm for using these newer deeper dissections. Moreover, coming into common usage were procedures on the platysma muscle, in which the lateral portion of the muscle is divided and then suspended, thereby tightening the still-undissected overlying skin. We also realized that a submental Z-plasty of the central platysmal bands could improve the anterior neck contour. This was associated with procedures of imbricating the subdermal soft tissues under the chin to correct the “witch’s chin” look. We also used selective chin augmentation to improve the acuteness of the cervical-mental angle. In 1985, after the introduction of cannulas for liposuction, we began to use small, flat, blunt cannulas as subcutaneous dissectors for the cheek and neck. This idea was introduced to me by one of our residents, Abram Nguyen (resident, 1985–87). This technique proved to be less traumatic than sharp dissection. We found that with gentle pressure, we could create subcutaneous tunnels in the proper plane that could later be connected by selective sharp dissection under direct vision.
The deep plane dissection techniques continued to evolve but those of us favoring a more conservative approach began to rely on plication of the SMAS layer and then began to use the lateral SMASectomy described at a meeting by Daniel Baker of New York City.[117] We were influenced by the article by Baker and Conley in 1979 about danger of facial nerve injuries during face-lifts.[118] It certainly encouraged us to take a more critical look at the deep plane approaches because of the possible injuries to branches of the facial nerve. Conley and Baker very clearly outlined the anatomy of the various branches of the nerve and how they might be damaged by some of the techniques in use at the time. The article described in detail the course of the ramus mandibularis of seventh nerve and showed it to be much more variable in its relation to the lower border of the mandible than the description in the study of preserved cadavers by Dingman and Grabb in 1961. This supported our conviction that it was essential to use fresh cadaver material for all our anatomic dissections for them to be reliably translated into clinical situations. We followed that in subsequent anatomic dissection projects. We also paid close attention to Peter McKenny’s description in 1980 of how to avoid injury to the greater auricular nerve in the posterior neck.[119]
In 1977, Dr. Ron Wexler published an article concerning possible incisions for male face-lifts based on the experience he gained while at Jackson Prison as a resident.[120] He emphasized the importance of and illustrated techniques to ensure leaving adequate hairless preauricular skin posterior to the sideburn at the end of the procedure.
Paul Dempsey (resident, 1976–78), who finished his training along with Eric Austad and Paul Izenberg, introduced to us in the early 1980s the concept of a subperiosteal approach through a coronal incision to elevate the midface. Dr. Izenberg and I did fresh cadaver dissections to help define the fascial relationships in the lateral forehead and dissection down to the zygomatic arch where a subperiosteal dissection over the zygoma allowed safe dissection and suspension of the deeper layers of the midface.
During those years, we became convinced that it was very important to determine whether the aging patient’s preoperative rationale for improvement was based on her own feelings of decreased self-esteem, or on wishing to have career advancement with a more youthful appearance. We found that these valid patient desires correlated well with the best assurance of a satisfied and happy patient postoperatively. This kind of preoperative assessment required a thorough pre-op interview using a lot of open-ended questions to ensure that the patient’s motivation was not based on something her husband or a friend might be insisting on. The value of this approach was confirmed by a study by Goin, Burgoyne, and Goin in 1980. They reported that a patient’s desire to “improve their self-image and to advance their career were reasonably reliable predictors of [postoperative] psychological improvement.”[121] In addition, we were always concerned about the effect of smoking in face-lift patients and usually required them to stop smoking for at least six weeks preoperatively. Our concern was confirmed by a study reported by Rees in 1984 that smoking did increase the risk for cheek flap vascular impairment postoperatively.[122] We also applied this rationale to operations such as abdominoplasty and even, in some cases, reduction mammoplasty and breast ptosis.
Brow-Lift and Blepharoplasty
Drs. Dingman, Peled, and Izenberg stated at the beginning of their article in 1979, “Forehead wrinkles, drooping eyebrows, and blepharoptosis are some of the earliest signs of the aging face. Loss of skin elasticity in conjunction with gravitational forces upon the forehead, brow, and upper lids results in a constant tired or angry expression. These changes are progressive . . . and do not reflect the patient’s true personality. Attempts to correct this condition by [eyelid] skin excision alone are usually ineffective and disappointing to both patient and surgeon.”[123]
This point of view reflected very accurately the importance that Dr. Dingman always stressed, with both the faculty and residents, of assessing the whole face whenever evaluating a patient’s aging changes. Seeing post-op patients with him in his office, we were witness to the profoundly positive effect that the alleviation of the “tired and angry” look had for the patient, often as, or more, important than improvement in the lower face. The article also discussed the various approaches for these corrections, including transcoronal, either through the scalp or at the hairline in cases of high foreheads in females or also under special circumstances, that is, a male patient, using a forehead wrinkle for access. These approaches were all eventually superseded by the endoscopic approach to forehead and brow-lifts.
We were all influenced by the seminal work presented by Dr. Salvador Castanares describing the anatomy of the herniated intraorbital fat and how to deal with it safely during blepharoplasty surgery. His work became the mainstay of our approach to “baggy eyelids.”[124] During those years, we were also constantly reevaluating our results with blepharoplasty. We gradually became more conservative about fat removal in the upper lids, except for the nasal fat compartment, in an effort to avoid the “hollowed out” look. We also removed less upper eyelid skin in conjunction with brow-lifts, thereby minimizing the risk of a post-op patient being unable to close his or her upper lids comfortably, especially at night. In the lower lids, we always had the patient (under local and light sedation) open his or her mouth widely before excising the lower lid skin. Using skin-muscle flaps and tightening the orbicularis laterally helped. These were the days before common use of canthopexy. We also repaired senile ptosis due to levator dehiscence, but routinely referred the congenital cases to pediatric ophthalmology. When patients presented as adults with an unclear history, a high school photograph was most helpful in ruling out whether the ptosis might be congenital. We were also on the lookout for cases of myasthenia gravis that can sometimes present with ptosis as an initial symptom to avoid surgery and make sure the patient received proper medical evaluation and treatment.
Mr. John Mustarde of Scotland was a good friend of Dr. Dingman’s and a visiting professor more than once, as well as a fishing companion. He graciously introduced all of us to the fundamental principles of reconstruction of resected and traumatized eyelids. His famous book Repair and Reconstruction in the Orbital Region[125] became a bible for us and provided a basis for dealing with many complex problems. We all felt deeply indebted to this very learned and charming Scotsman (Photo 56).
Liposuction
Although the use of suction and a sharp curette to remove subcutaneous fat was first published in PRS in 1978,[126] this idea did not receive much attention until, in 1983, Dr. Illouz of France published his results of lipolysis in three thousand cases, having performed it since 1977.[127] There was a lot of skepticism about the technique in the United States, but finally the ASPRS appointed a committee to evaluate the procedure. A group of plastic surgeons went over to France and came back with rather glowing reports after spending time with Dr. Illouz and evaluating his work. There were arguments whether the technique should be wet or dry, but the wet technique of Dr. Illouz seemed to have less bleeding associated with it.
There were some postgraduate teaching conferences on liposuction set up by respected plastic surgeons, and several of our faculty and residents attended these training sessions. Many of us became convinced that the technique, as described by Dr. Illouz, was effective with minimal morbidity in the removal of localized collections of unaesthetic subcutaneous fat accumulation. It seemed to be particularly useful in the lateral hips, abdomen, and in the submental area of the neck. We were also sure that this was not indicated as a substitute method of weight loss in truly obese patients.
The worry about overcorrection was frequently an issue with the then available large diameter cannulas. As small diameter blunt cannulas became available, they allowed much more finesse in removal of fat and avoided getting too superficial where it might result in rippling under the skin, one of the complications of this type of procedure. When the tumescent technique of infiltrating large volumes of saline containing very dilute local anesthesia and very dilute epinephrine was introduced, it became possible to carry out the procedure in an ambulatory setting with supplemental IV sedation. With greater experience, we learned some of the limitations of this procedure early on and tended to avoid the medial thighs, because the skin did not contract well. We had some good results, and ultimately, we were able to combine lateral flank liposuction together with the standard abdominoplasty, but avoiding suction in the abdominal flap. It is interesting to note that currently some type of liposuction is the most common aesthetic operation performed in this country. Of course, liposuction has gone on to much wider use, including collecting fat for autogenous fat transplantation.
Facial Fractures
With the publication of their book, Surgery of Facial Fractures, in 1964,[128] Drs. Dingman and Natvig brought the fundamentals of caring for these injuries to our attention in Ann Arbor, as well as to the plastic surgery community and the world beyond. It bears mention that Dr. Child, whose political skills supported the development and formation of the Section of Plastic Surgery in the years leading up to the publication, wrote the foreword to the book. It is only speculation, but one wonders if the high regard for the authors expressed in the foreword reflected his expectation of Dr. Dingman’s potential as a great teacher. Quoting Dr. Child, “The text by Doctors Dingman and Natvig encompasses past and present knowledge of facial fractures. It clearly points the way toward a better understanding of one of surgery’s most humanitarian accomplishments: the mending of fractured faces . . . the authors . . . have captured the past with charm and the present with vigor. The ability to teach their skills by word and by picture is limited only by their student’s capacity and motivation to learn.”[129]
The book’s publication preceded by two years President Lyndon Johnson’s signing the law making automobile seat belts mandatory in 1968. However, it was not until the mid-1970s, when the development of the enhanced seatbelt reminder (ESBR) became universal and seatbelt usage increased from around 15 percent to 70 percent, that these serious facial injuries saw a marked reduction. So for the first ten years after publication, all types of facial fractures were always a big part of our practice. Dingman and Natvig’s book provided a description of types of injuries for each anatomic location as well as step-by-step illustrations of the necessary corrective maneuvers, and the book became an essential guide for surgical correction. The authors described and illustrated the use of the internal suspension sutures from a stable frontal bone to facilitate intraoral suspension of the maxilla so necessary in the management of those complex cases of skull-facial bone disjunction. The epilogue of the book was written by G. Kasten Tallmadge, a colleague of Dr. Natvig’s at Marquette Medical School and a friend of Dr. Dingman’s. It is a beautiful description of the part of the body so intrinsic to the art of plastic surgery. Quoting in part, “Is there another part of the body which is more nearly one’s self than the face? The incredibly complex arrangements of the parts from the microscopic to the massive have given to man alone . . . the power of intelligible speech and the great gift of his ability to make music. The ceaselessly mobile lines of the countenance conceal or reveal the recondite workings of the spirit.”[130]
Although car safety improvements significantly reduced their incidence, facial fractures resulting from a variety of other etiologies remained a significant part of our trauma care. As an aside, I learned the hard way about the importance of knowledge of pre-op occlusion in conjunction with a case of a patient with LeFort II midface fractures along with mandible fracture. At operation, we applied the direct interosseous wiring and suspension wiring techniques. I got the displaced structures back into what I thought was very nice occlusion. By the next morning, the patient had pushed his lower jaw forward and was in a Class III relationship. I was confused by it but his mother said, “You know I think he may have always looked like that.” Because the patient lived locally, we contacted his dentist and obtained his pre-op dental impressions and realized that he was prognathic before the injury. It was a good lesson to me to always know as much as you can about a patient preoperatively.
Naso-orbital fractures were one specific type of severe facial fractures not discussed in great detail in Drs. Dingman and Natvig’s book. Early on, we recognized the uniqueness of these complex injuries from a frontal force pushing the nasal mass posteriorly into the lacrimal-ethmoid structures. Associated fractures of the frontal sinus often involved the posterior wall and the anterior cranial fossa. To achieve an accurate diagnosis, the clinical exam and regular x-rays had to be followed by open reduction. Direct wire fixation was necessary after reducing all the displaced fragments, along with both neurosurgical and ENT consultation for management of the frontal sinus fractures unless the injury was restricted to the anterior wall of the sinus. By 1969, Drs. Dingman, Grabb, and I had gained enough experience with these cases to report our findings in Archives of Surgery.[131] In 1979, Drs. Dingman and Izenberg contributed a comprehensive review of the possible complications of facial fractures as well as facial soft tissue injuries in John Conley’s textbook on head and neck surgery complications.[132]
Maxillofacial Surgery
This was a special interest of Dr. Dingman’s from the beginning of his career. We were all intrigued by the collaboration that had developed between him and an inventive orthodontist in Ann Arbor, Dr. Robert Ponitz, DDS (Photo 57). Once a decision was made as to whether the maxilla, mandible, or both were to be modified, Dr. Ponitz made models and splints to show exactly what had to be done and what the exact dental occlusion was to be postoperatively. He excelled in doing preoperative orthodontics so that when the surgery was done, the teeth fit nicely together in the most desirable occlusal relationship. This collaboration had led to the evolution of quite sophisticated planning and immobilization techniques. For those of us who were not trained in dentistry and the fundamentals of dental occlusion, it was a bit of an uphill battle to learn these concepts, but by working closely with Dr. Dingman, we were encouraged to branch out on our own with the help of his close friend and colleague, Dr. Ponitz. Many of our maxillofacial patients were adults with secondary cleft lip sequelae who had orthognathic deformities. Before the sagittal split mandibular procedure achieved popularity (it was never favored by either ROD or Dr. Ponitz), we performed vertical ramus osteotomies or body ostectomies for mandibular setback. We would often go to Dr. Ponitz’s office before the case and go over the cephalograms and the models, and look at the splints. He was extremely cooperative and so interested in the actual surgery that he would join us in the OR. As he was a member of the medical staff at SJMH, he could scrub in and assist us, and his on-the-spot analysis was always helpful.
An interesting example of this collaboration with Dr. Ponitz to solve a unique clinical problem was published in 1969 in PRS.[133] The article referred to a previous successful case (from another institution) of functional sternocleidomastoid muscle (SCM) transfer to treat masseter muscle atrophy secondary to poliomyelitis. The patient in the current article had congenital absence of bilateral masseter muscles. Seen as a teenager, he was unable to actively close his mouth, having to position his chin on his chest and push his head forward to achieve closure. He also had a massive open-bite deformity and absence of mandibular angles. Two mandibular body osteotomies and iliac bone grafts were required to reshape the mandible. Both SCM muscles were transferred on pedicles containing the accessory nerve and occipital artery. They were positioned between the zygoma and lower border of the mandible. Both transferred muscles regained their function and allowed the patient to actively close his mouth. Dr. Hudak related to me his experience with another application of transferring the SCM:
Looking back, I had the opportunity as a resident to do a facial reconstruction using the sternocleidomastoid muscle for facial atrophy. That procedure done in 1969 was a forerunner of many of those procedures that followed using muscle for breast reconstruction as well as free muscle grafts. I wrote a paper on the case: Sternocleidomastoid Flap for Correction of Soft Tissue Defects of the Face which I presented at the National Residents Conference in Salt Lake City in 1970. I won the award for the Outstanding Paper for Head and Neck Reconstruction. I think this was one of the first muscle trans-positioning papers.[134]
Dr. Dingman, over the years, attracted many patients who were suffering from malfunction of the TMJs from a variety of causes. Many of them were severely symptomatic. He worked out a safe surgical approach to both the upper and lower aspects of the joint, allowing him to refine his surgical procedures. He reported his surgical results in two articles: the first with 440 cases in 1969 coauthored with a resident at the time, Dr. Eric Constant,[135] and the second in 1975, coauthored with another resident, Dr. Richard Lawrence and Dr. Dingman’s son David, a plastic surgeon on the faculty of the University of Utah, reporting on another twenty-five cases.[136] The surgical approach to the TMJ was thoroughly described and illustrated. A significant proportion of the patients benefited from the variety of surgical procedures.
During these years and subsequently, it was discovered that one of the main underlying etiologic factors leading to dysfunction of the TMJ was unresolved traumatic dental occlusion. Recognition and correction of it as well as prevention of the resulting chronic stress on the TMJ, with a removable dental splint worn at night, ultimately resulted in a significant diminution of the difficult cases. After being contacted by the authors for comment about the TMJ reports, Dave Dingman replied,
It is a hard subject to discuss. The real cases such as the originals and some of mine had real pathology. I chased many of these cases down by flying around the country and the results were dramatic, with normal pain free function decades after the surgery. One such case was a girl from Honduras that had bilateral ankyloses. It was a big deal in the local papers as it was an Interplast case and the child stayed a while and became friends with my twins. She did not want to leave. Some of the bad results were patients who had prosthetic joint implants. Some got infected and the results were not good. I am not sure about the role good dentistry played [in prevention of TMJ dysfunction] but I am sure it was a factor. Some of the reduction in the number of surgical cases may have been due to alleviation of the secondary TMJ symptoms in depressed patients when treated with new and better anti-depressant medication.[137]
Dr. Dingman also did a lot of other types of maxillary surgery, and there were always the possibility of risks. Dr. Lenny Glass (1968–70) recounted such a tragic case during his time as a resident, “I remember so vividly the teenager who exsanguinated in his hospital bed apparently from iatrogenic damage to his carotid artery (a rare complication) which occurred five to six days previously during one of ROD’s first LeFort II advancements. I never forgot that. A curved osteotome was used to blindly separate the perpendicular plate from the maxilla. Seemed too risky a procedure to do without visualization so I never did one.[138] It is probably important to emphasize the dangers and risks faced by pioneers like Dr. Dingman in performing these difficult procedures for the first time. We do not always remember the debt we owe to these innovators as these complex operations can now be performed much more safely on a routine basis.
Another recollection came from Bruce Novark (1972–74) about ROD’s open-mindedness in regard to residents’ assessments of a problem:
As renowned an icon as he was, he would always listen to our input and critiques. We saw a 26-year-old woman in clinic with an apparent prominent mandible and class III malocclusion. The chief thought she needed mandibular surgery, I wasn’t so sure. Further clinical exam and cephalometric analysis demonstrated the problem to be in the midface. I presented my evidence and belief that she needed a LeFort II naso-maxillary advancement. (Don’t think one had been done at the U of M at that time.) The boss listened, agreed (he didn’t say, “no we’ll do it my way”), and I went ahead with the procedure with his assistance. ROD was that kind of guy.[139]
Lip Reconstruction
Dr. Dingman demonstrated his characteristic interest in innovation in two instances with ideas suggested by residents. One was with Ron Wexler (1970–72) in reporting a case of a large subtotal excision of the lower lip excluding the commissures in an older patient with a redundant upper lip. The article described two Stein-Eslander Abbe style flaps that were designed to extend up just lateral to the nose and rotated into the lower lip on skeletonized superior labial arterial pedicles. These were divided in a couple of weeks providing adequate functioning lip balance in a difficult situation.[140] The other instance was with Grant Fairbanks (1969–71), who thus described it:
Dr. Dingman was always interested in publishing new techniques if he thought it could benefit the patients and the profession. On one occasion, I was recreating the oral commissure on a woman who had lost this portion of her lip in an auto accident. Dr. Dingman came in and looked over our shoulders and asked me what I was doing. I explained the approach and stated that I had performed a similar procedure while serving on the Hospital Ship Hope in Tunis which Dr. Dingman had arranged for me through his friend at Sinai Hospital in Detroit, Herbert Bloom, DDS. He told me this was a new procedure and it should be presented.[141] (It was presented at ASPRS 1971 and published in PRS in 1972.[142])
Introduction of Craniofacial Surgery to Michigan
Dr. Haskell Newman related in detail the sequence of events that brought craniofacial surgery to Michigan and its subsequent developments:
In 1967, Dr. Paul Tessier presented the first successful case in which a facial advancement was performed to treat a patient with Crouzon Syndrome. A publication described the technique.[143] A Le Fort III osteotomy advancement was designed to include a major portion of the orbits with the upper jaw. This technique of dissecting tissue from the facial bones with simultaneous intracranial exposure and circumferential mobilization of the orbits enabled repositioning of the eyes and the skull. Tessier’s techniques are based on the principle that skull and facial bones must be repositioned or reconstructed before soft tissue can be repaired. In the early 1970s, surgeons from the United States visited Tessier to learn the new operative techniques. Among his early students was Reed Dingman, Chairman of the Section of Plastic Surgery at the University of Michigan.[144]
Dr. Glass (1970–71) also recalled that “as soon as Paul Tessier (from France) told the world about his work, ROD had the guts, interest, and stamina to embrace this new discipline called craniofacial surgery and started doing cases.”[145] Dr. Newman continued, “In 1970, Dr Dingman initiated craniofacial surgery at the university when he accomplished the surgical correction of a patient with Crouzon craniofacial deformity assisted by a university neurosurgeon and Dr. John Converse, the chairman of plastic surgery, New York University”[146] (Photo 58). This event was remembered by Dr. Hudak who recounted, “One of our most interesting visiting professors was John Converse. We had the opportunity to see him and Dr. Dingman operate on a child with Crouzon Syndrome. That was a unique experience for all who witnessed the operation. It took place in the year 1969–70, which was my second year as resident.”[147] Dr. Wexler recollected a subsequent case that took place in 1972.[148]
Dr. Newman recalled,
In the fall of that year, I was an assistant professor in the Department of Otolaryngology and I applied for a residency position in plastic surgery. The interview with Dr. Dingman centered on a joint interest in the developing specialty of craniofacial surgery. After completing the residency in plastic surgery, encouraged by Drs. Dingman and Grabb, I completed a fellowship of craniofacial surgery with Dr. Ian Munro at the University of Toronto, and in 1978, I was recruited by Dr. Grabb to establish a multidisciplinary team to provide consistency and safety with the most advanced treatment for patients suffering from craniofacial anomalies.
The plastic surgery faculty with interest and training in the correction of craniofacial anomalies was expanded in 1981 with the addition of Dr. Lou Argenta following his fellowship training with Dr. Tessier in Paris [Photo 59]. In 1982, Dr. Newman confined his practice to SJMH and Dr. Argenta directed the craniofacial program at the University of Michigan Hospital systems until his departure in 1988. Adult craniofacial and maxillofacial surgery and specialty training were expanded in practices at the university hospital and SJMH. Neurosurgical participation in the craniofacial program was enhanced when, in 1983, Dr. Joan Venes was recruited to establish and head a division of pediatric neurosurgery. Through the combined interest and efforts of Dr. Venes and the plastic surgery faculty, craniofacial surgical focus expanded to include early interventional correction of craniofacial deformities in infants and young children.[149]









