Sign-Tracking and Drug Addiction
Skip other details (including permanent urls, DOI, citation information): This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. Please contact [email protected] to use this work in a way not covered by the license.
For more information, read Michigan Publishing's access and usage policy.
Chapter 3: The Neurobiological Mechanisms Underlying Sign-Tracking Behavior
aNeuroscience Graduate Program
bDepartment of Psychiatry
cMolecular and Behavioral Neuroscience Institute, the University of Michigan, Ann Arbor, MI 48109
Corresponding Author:
Shelly B. Flagel, PhD, 205 Zina Pitcher Place, Ann Arbor, Michigan, 48109-0720; e-mail: [email protected]; phone: 734-936-2033; fax: 734-647-4130.
Introduction
Associative learning strategies are often advantageous as they result in the recognition of cues in the environment that reliably predict resources needed for survival, such as food. These cue-reward associations are partially governed by Pavlovian learning processes, whereby a cue in the environment that precedes the delivery of a reward (unconditioned stimulus, US) becomes a conditioned stimulus (CS). While a CS has predictive value, it can also acquire incentive motivational value, thereby being transformed into an incentive stimulus or a “motivational magnet” (Robinson & Berridge, 1993). This process is known as incentive salience attribution and is believed to contribute to addiction (Berridge & Robinson, 2016; Robinson & Berridge, 1993). For example, cues in the environment (people, places, paraphernalia) previously associated with the drug-taking experience can become incentive stimuli and gain excessive control over behavior. Exposure to these stimuli, therefore, can elicit drug-seeking and drug-taking behavior and cause one to relapse in spite of the desire to remain abstinent. Thus, a better understanding of the neural processes underlying incentive salience attribution may lead to more effective treatments for addiction and the prevention of relapse.
To elucidate the neurobiological mechanisms underlying incentive salience attribution, we use an animal model that allows us to dissociate the predictive value from the incentive motivational value of a reward cue. This model, known as the sign-tracker (ST)/ goal-tracker (GT) model, is illustrated in Figure 3.1 and described in greater detail in Chapter 1 of this book. While STs and GTs differ in their conditioned responses (CR), both phenotypes learn their respective CRs at the same rate and consume the food reward. Additionally, compared to GTs, STs work harder for the presentation of the lever-cue in the absence of the food reward (Robinson & Flagel, 2009). Thus, while the lever-cue is a predictor and elicits a conditioned response for both STs and GTs, only for STs does it also become an incentive stimulus.
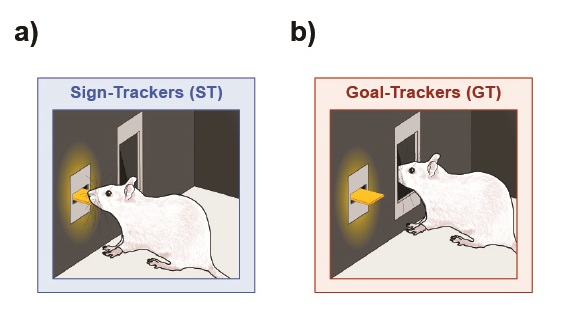
Importantly, sign-trackers attribute enhanced incentive motivational value to both food- and drug-associated cues and will sign-track to discrete cues associated with cocaine (Yager & Robinson, 2013) and opioids (Yager, Pitchers, Flagel, & Robinson, 2015). In addition, relative to GTs, STs have been found to work harder for the delivery of cocaine (Saunders & Robinson, 2011) and show greater drug- and cue-induced drug-seeking behavior, or enhanced propensity for relapse, following limited drug exposure and a period of abstinence (Saunders & Robinson, 2010; Saunders, Yager, & Robinson, 2013; see also Robinson et al., this volume). The sign-tracker/goal-tracker animal model, therefore, supports the long-standing notion that Pavlovian incentive learning processes contribute to addiction-related behaviors (Bindra, 1978; Bolles, 1972; Robinson & Berridge, 1993; Stewart, de Wit, & Eikelboom, 1984; Toates, 1981) and provides a means to parse the underlying neurobiological mechanisms. The remainder of this chapter will highlight the neural circuits and associated neural processes believed to play a role in the propensity to attribute incentive motivational value to reward cues.
Neurobiology of Motivated Behavior
The brain is structurally and functionally divided into several regions, some of which communicate directly with one another through the synaptic connections of neurons (see Figure 3.2). Neurons can become activated in response to stimuli in the outside world, such as cues that are associated with rewards, and/or in response to chemical signals released into its surrounding environment in the brain. When a neuron becomes activated, it causes a cascade of reactions within the cell such as the production of gene transcripts and proteins, which can be measured. For example, c-fos, an immediate early gene that is “immediately” produced upon neuronal activation, can be quantified in specific brain regions and used to determine the activity of said brain region in response to certain stimuli or in association with a given behavior (for review see Kovacs, 1998). Additionally, when neurons become activated, they release various chemicals that can bind to receptors on surrounding cells, thereby influencing the activity of neighboring neurons. These chemicals, such as dopamine and acetylcholine, can be measured, and their fluctuations associated with different behaviors. Indeed, such chemicals have distinct effects in different brain regions, so examining the chemical profile of a given brain region in response to environmental stimuli can further our understanding of how that region is contributing to behavior.
Brain regions can work in concert with one another, creating a neurocircuit that mediates behavior (Figure 3.2). The “motive circuit” is a set of cortical and subcortical nuclei that integrate information regarding a motivationally salient event, such as the presentation of a reward cue, and guide subsequent behavior (Kalivas & Volkow, 2005). Cortical structures, such as the prefrontal cortex (PFC), govern executive functions in the brain (for review see Diamond, 2013; Fuster, 2001; Jurado & Rosselli, 2007; Nyberg, 2018). The PFC is believed to exert “inhibitory control,” allowing one to attend only to the most meaningful stimuli and thereby mediate goal-directed behavior (for review see Asplund, Todd, Snyder, & Marois, 2010; Mihindou, Guillem, Navailles, Vouillac, & Ahmed, 2013; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). For example, students need to rely on this “inhibitory control” exerted by the PFC in order to focus on what they are learning in class, perhaps in lieu of attending to their phone. In contrast to cortical structures, subcortical structures tend to mediate aspects of emotions such as fear and reward (for review see Baxter & Murray, 2002; Davis, 1992; Shin & Liberzon, 2010), autonomic functions such as hunger and sleep (Dietrich & Horvath, 2013; for review see Salin-Pascual, Gerashchenko, Greco, Blanco-Centurion, & Shiromani, 2001) and different forms of learning (for review see Baxter & Murray, 2002; Daniel & Pollmann, 2014; Liljeholm & O’Doherty, 2012). Nuclei throughout the cortical and subcortical components of the motive circuit communicate with one another to mediate various aspects of motivated behavior, ranging from encoding the value of the reward to determining the correct behavioral output to obtain that reward (for review see Kalivas & Volkow, 2005). Thus, it is not surprising that dysregulation of this circuit contributes to addiction and relapse (Kalivas & Volkow, 2005).
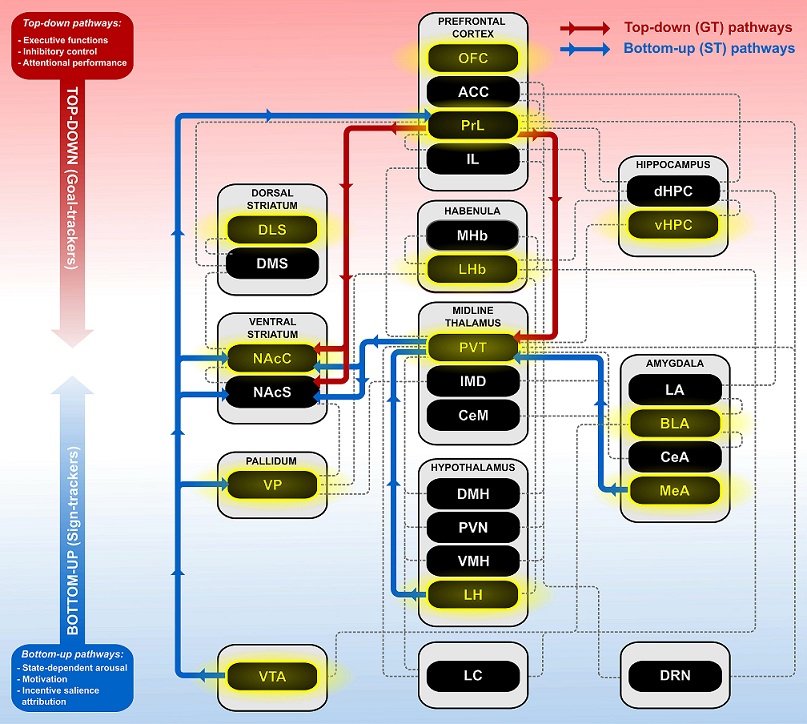
STs and GTs differ in the extent to which they rely on the motive circuit, such that, in response to a discrete food- or drug-associated cue, STs show greater neuronal activation (i.e., c-fos expression) throughout this circuit (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Yager, Pitchers, Flagel, & Robinson, 2015). That is, only when the reward cue is attributed with incentive salience (i.e., in STs) does it activate the cortico-striatal-thalamic motive circuit. Furthermore, when patterns of neuronal activity were examined between brain regions for a given phenotype, correlated activity was found between the cortical and subcortical areas for GTs, whereas for STs the correlated patterns of activity were restricted to subcortical regions (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a). As reviewed later in the text, these data, as well as more recent findings (Flagel & Robinson, 2017; Haight, Fuller, Fraser, & Flagel, 2017; Sarter & Phillips, 2018), suggest that GTs rely on “top-down” cortical processes to inhibit the propensity to attribute incentive salience to reward cues; whereas enhanced “bottom-up” subcortical drive in STs increase that propensity.
“Top-Down” Cortical Control
Goal-trackers are believed to have greater top-down attentional control than STs, and it has been postulated that a “deficit” in this top-down control contributes to the sign-tracking phenotype (for review see Sarter & Phillips, 2018). In support, GTs perform better than STs on tasks that demand more cortical control, including those associated with impulse control (Flagel, Robinson, Clark, Clinton, Watson, Seeman, Phillips, & Akil, 2010; Lovic, Saunders, Yager, & Robinson, 2011) and sustained attention (Paolone, Angelakos, Meyer, Robinson, & Sarter, 2013). Next we will review the neurobiological mechanisms that have been associated with this proposed “deficit” in cortical control and attentional processing in sign-trackers and discuss the implications of these findings in regards to addiction.
Prefrontal Cortex
Role of Cortical Cholinergic Activity in Sign-Trackers and Goal-Trackers
As previously discussed, the PFC mediates top-down executive control in the brain, thereby maintaining goal-directed behaviors (Asplund, Todd, Snyder, & Marois, 2010; Mihindou, Guillem, Navailles, Vouillac, & Ahmed, 2013; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). That is, the PFC acts to selectively guide attention such that the focus is on the task at hand and not on “irrelevant” cues in the surrounding environment. The ability to maintain performance on tasks that demand sustained attention is associated with increases in acetylcholine (ACh) activity within the PFC (St Peters, Demeter, Lustig, Bruno, & Sarter, 2011). On such a task, STs show poor performance relative to GTs and concomitantly exhibit attenuated task-related increases in PFC ACh levels (Paolone, Angelakos, Meyer, Robinson, & Sarter, 2013). Furthermore, when an ACh receptor agonist is administered, thereby increasing ACh levels within the brain, STs show improvement on attentional performance (Paolone, Angelakos, Meyer, Robinson, & Sarter, 2013). Thus, it is believed that lower attentional performance in STs is due to lower levels of cortical ACh compared to GTs (Paolone, Angelakos, Meyer, Robinson, & Sarter, 2013). Importantly, however, the differences in ACh levels between STs and GTs are not apparent under baseline conditions and only evident under stimulated conditions, such as task-performance (Paolone, Angelakos, Meyer, Robinson, & Sarter, 2013). Such deficits in cortical cholinergic modulation in STs have recently been attributed to the inability to transport choline, the precursor of ACh, into the neurons that produce and release it (Koshy Cherian, Kucinski, Pitchers, Yegla, Parikh, Kim, Valuskova, Gurnani, Lindsley, Blakely, & Sarter, 2017). In support, when the transport of choline into neurons is blocked early in Pavlovian training, sign-tracking behavior increases while goal-tracking behavior decreases (Koshy Cherian, Kucinski, Pitchers, Yegla, Parikh, Kim, Valuskova, Gurnani, Lindsley, Blakely, & Sarter, 2017). Thus, it has been suggested that the lack of top-down executive control in STs is, at least in part, driven by inefficient mechanisms at the cortical cholinergic transporter, resulting in a bias for bottom-up or stimulus-driven attention (Koshy Cherian, Kucinski, Pitchers, Yegla, Parikh, Kim, Valuskova, Gurnani, Lindsley, Blakely, & Sarter, 2017).
Cortical ACh levels are also differentially affected by the presentation of a Pavlovian cocaine cue in STs versus GTs, as are cortical dopamine levels (Pitchers, Kane, Kim, Robinson, & Sarter, 2017). In STs, cocaine cue presentations elicit approach behavior and elevate dopamine levels in the PFC, while ACh levels remain unchanged. Interestingly, cue-elicited increases in dopamine levels correlate with higher levels of approach to the cocaine cue (Pitchers, Kane, Kim, Robinson, & Sarter, 2017), suggesting that cortical dopamine plays a role in encoding the incentive motivational value of the cue (but see also Ellwood, Patel, Wadia, Lee, Liptak, Bender, & Sohal, 2017). Conversely, in GTs, presentation of the cocaine cue does not elicit approach and does not affect dopamine levels but does increase ACh levels (Pitchers, Kane, Kim, Robinson, & Sarter, 2017). Importantly, PFC ACh levels are not correlated with cue-elicited behaviors. These data highlight the involvement of distinct cortical processes in regulating the behavior of STs and GTs and demonstrate a role for PFC dopamine levels in mediating the incentive motivational value of reward cues.
Conversely, the lack of cue-elicited changes in cortical dopamine levels, concurrent with increased ACh, support the notion that GTs rely on dopamine-independent cognitive processes to encode the meaning or value of reward cues (Dickinson & Belleine, 2002; Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b; Sarter & Phillips, 2018). That is, for GTs, as a function of enhanced cortical processing, a discrete reward cue is merely an “informational” stimulus that is relatively devoid of incentive properties (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a). Thus, GTs exhibit goal-directed approach to the location of impending reward delivery if the reward cue is food and explicitly do not approach drug-associated cues when no alternative behavioral response is available (i.e., when the drug reward is delivered intravenously). Furthermore, these distinct cortical processes inherent to sign- and goal-trackers are believed to contribute to the enhanced attention to contextual cues that is characteristic of GTs (Pitchers, Phillips, Jones, Robinson, & Sarter, 2017b; Saunders, O’Donnell, Aurbach, & Robinson, 2014).
Contexts and discriminative stimuli are powerful motivators for drug relapse and are inherently more complex than a discrete cue signaling reward availability. Indeed, a context is defined as an environment where no single cue predicts reward availability but rather the compilation of cues together does so. For example, the environmental context of a bar includes the smell of alcohol, the sight of frosty mugs, the sound of music, the presence of friends, and so on Discriminative stimuli within this context may include the flashing neon sign indicating the bar is “open”; that is, a cue that signifies whether or not the presentation of a subsequent cue will be followed by a reward. Given the complexity of such stimuli, it is perhaps not surprising that GTs seem to be more responsive to contextual cues and discriminative stimuli (Pitchers, Phillips, Jones, Robinson, & Sarter, 2017b; Saunders, O’Donnell, Aurbach, & Robinson, 2014). In fact, GTs show greater drug-seeking behavior upon exposure to drug-associated contexts (Saunders, O’Donnell, Aurbach, & Robinson, 2014) or discriminative stimuli previously associated with drug availability (Pitchers, Phillips, Jones, Robinson, & Sarter, 2017b). Moreover, when cholinergic transmission is attenuated in the PFC, GTs no longer show higher rates of drug-seeking behavior compared to STs in the presence of discriminative stimuli (Pitchers, Phillips, Jones, Robinson, & Sarter, 2017b). Thus, while elevated cortical ACh levels appear to inhibit the attribution of incentive motivational value to a cocaine cue, these same processes appear to make GTs more vulnerable to context-induced relapse. These data suggest that both STs and GTs are vulnerable to addiction (Kawa, Bentzley, & Robinson, 2016), or relapse (Kuhn, Klumpner, Covelo, Campus, & Flagel, 2017; Pitchers, Phillips, Jones, Robinson, & Sarter, 2017b; Saunders, O’Donnell, Aurbach, & Robinson, 2014; Saunders & Robinson, 2011; Saunders, Yager, & Robinson, 2013) but via different psychological and neurobiological pathways.
Role of Serotonin in Pavlovian Conditioned Approach Behavior and Incentive Motivational Learning
In addition to acetylcholine, serotonin levels in the PFC have also been shown to play a role in sign-tracking behavior (Campus, Accoto, Maiolati, Latagliata, & Orsini, 2016; Winstanley, Dalley, Theobald, & Robbins, 2004). Serotonergic neurons originate in the dorsal raphe nucleus and project widely throughout the brain, including to the PFC (Michelsen, Prickaerts, & Steinbusch, 2008; Vertes, 1991). Serotonin has a wide range of functions within the brain, including mediating mood and appetite (for review see Mohammad-Zadeh, Moses, & Gwaltney-Brant, 2008). Increases in serotonin levels in the PFC, but not striatum, have been reported following Pavlovian training, therefore demonstrating a role of PFC serotonin in appetitive Pavlovian tasks (Tomie, Tirado, Yu, & Pohorecky, 2004). Furthermore, Pavlovian training results in an increase in the binding of serotonin to the serotonin 1a and 2a receptors within the PFC (Tomie, Di Poce, Aguado, Janes, Benjamin, & Pohorecky, 2003). When serotonin is depleted from the forebrain, rats approach a reward cue (i.e., sign-track) more often and do so faster compared to control rats (Winstanley, Dalley, Theobald, & Robbins, 2004). Additionally, when approaches to the reward cue no longer result in delivery of the food reward, rats with serotonin depletion continue to approach the reward cue to a greater extent than control rats (Winstanley, Dalley, Theobald, & Robbins, 2004). These data suggest that serotonin is not only contributing to the appetitive learning associated with Pavlovian training but also to the incentive motivational value of the reward cue. In agreement with these findings, it was found that depleting serotonin specifically within the medial PFC increases sign-tracking behaviors in mice (Campus, Accoto, Maiolati, Latagliata, & Orsini, 2016). Taken together, these data support a role of serotonin transmission within the PFC in mediating aspects of incentive motivational learning (Pentkowski, Duke, Weber, Pockros, Teer, Hamilton, Thiel, & Neisewander, 2010) and addiction-related behaviors (Anastasio, Liu, Maili, Swinford, Lane, Fox, Hamon, Nielsen, Cunningham, & Moeller, 2014; Swinford-Jackson, Anastasio, Fox, Stutz, & Cunningham, 2016).
“Bottom-Up” Subcortical Control
Whereas goal-trackers are thought to rely primarily on “top-down” cortical mechanisms to guide their goal-directed behaviors, sign-trackers are believed to have enhanced “bottom-up” processing, as a function of increased activity in subcortical regions, including the striatum, amygdala, midline thalamus, and hypothalamus (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Haight, Fuller, Fraser, & Flagel, 2017; Sarter & Phillips, 2018). Moreover, cue-induced activity is correlated only between subcortical regions in sign-trackers, such that activity in midline thalamic nuclei correlates with neuronal activity in the ventral striatum (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Haight, Fuller, Fraser, & Flagel, 2017). Next we highlight some of the subcortical regions and associated neural mechanisms that appear to play an important role in mediating the propensity to attribute incentive salience to reward cues (see also Figure 3.2).
Striatum: Dopaminergic Regulation of Incentive Motivational Learning
Ventral Striatum
The nucleus accumbens (NAc), a region within the ventral striatum, is a key component of the motive circuit (Kalivas & Volkow, 2005). The NAc receives dense dopaminergic projections from the ventral tegmental area, and this pathway, known as the mesolimbic pathway, plays an important role in reward-related processes (for review see Salamone & Correa, 2012; Volkow, Wise, & Baler, 2017). Over the course of Pavlovian learning, STs and GTs show differences in the mesolimbic dopamine system, with emergent differences in gene expression (Flagel, Watson, Robinson, & Akil, 2007) and distinct patterns of phasic dopamine release in the nucleus accumbens (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b). Phasic dopamine transmission in the NAc is known to be triggered initially by the receipt of a reward-US, but then, upon learning an association between a cue-CS and reward-US, the dopamine response shifts to the predictive-cue-CS (Day, Roitman, Wightman, & Carelli, 2007; Schultz, Dayan, & Montague, 1997). This pattern of dopamine activity is believed to support the “prediction error theory”; that is, that dopamine is acting primarily to encode the discrepancy between rewards received and those predicted (Montague, Dayan, & Sejnowski, 1996; Waelti, Dickinson, & Schultz, 2001). Thus, an unpredicted reward initially elicits an increase in dopamine activity, or positive prediction error; a fully predicted reward elicits no response to the reward itself; and the omission of a predictive reward results in a decrease in dopamine activity, or negative prediction error (Schultz, Dayan, & Montague, 1997). The prediction error theory, therefore, suggests that dopamine is used to update the predictive value of stimuli during associative learning and thereby guide cue-elicited behaviors (Balleine, Daw, & O’Doherty, 2008).
In contrast to the prediction error theory, others have long postulated that dopamine acts to encode the incentive motivational value of reward cues (Berridge, 2007; Berridge & Robinson, 1998). Until the advent of the sign-tracker/goal-tracker model, however, it was difficult to parse the processes underlying predictive versus incentive learning, as the two were confounded in the majority of studies (for review see Robinson, Yager, Cogan, & Saunders, 2014). Thus, the sign-tracker/goal-tracker model was exploited to address the long-standing debate in the field regarding the role of dopamine in reward learning. Using fast-scan cyclic voltammetry, which allowed the detection of dopamine on a sub-second time scale, Flagel, Clark and colleagues examined phasic dopamine release in the core subregion of the nucleus accumbens (NAcC) in response to cue and reward presentation in STs and GTs during Pavlovian training (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b). The NAcC was examined as it is considered a central locus for the dopamine-mediated effects of Pavlovian learning (Dalley, Laane, Theobald, Armstrong, Corlett, Chudasama, & Robbins, 2005; Di Ciano, Cardinal, Cowell, Little, & Everitt, 2001; Parkinson, Dalley, Cardinal, Bamford, Fehnert, Lachenal, Rudarakanchana, Halkerston, Robbins, & Everitt, 2002; Parkinson, Olmstead, Burns, Robbins, & Everitt, 1999). Flagel, Clark, and colleagues found that the “classic” prediction-error shift in dopamine from the reward-US to the cue-CS occurs only in STs. That is, in GTs, the dopamine response does not differ between cue and reward presentation over the course of learning. Given that the reward cue (CS) is a predictor and elicits a conditioned response for both STs and GTs, these data demonstrate that the shift in phasic dopamine must be encoding the incentive value of the cue and not the predictive value (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b). In support, when dopamine transmission is blocked via systemic administration of flupenthixol, a nonselective dopamine antagonist, the learning and expression of a sign-tracking response, but not goal-tracking, is attenuated (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b). A subsequent study expanded upon these findings demonstrating specifically that dopamine in the NAcC is necessary for the expression of sign-tracking and not goal-tracking behavior (Saunders & Robinson, 2012). Thus, sign-tracking is dopamine-dependent, and dopamine in the nucleus accumbens appears to be critical for incentive learning processes.
Dopamine transmission within the NAcC also plays an important role in individual variation in the propensity to relapse, or cue-induced reinstatement (Saunders, Yager, & Robinson, 2013). During a test for cue-induced reinstatement, the action (e.g., nose poke) that previously resulted in the presentation of the drug and associated drug-paired cue, now results in cue presentation without drug delivery. Thus, rats are responding based on the conditioned reinforcing properties of the drug-associated cue, which is thought to be akin to humans reporting craving in response to presentation of drug-associated images (e.g., paraphernalia; Childress, Ehrman, McLellan, & O’Brien, 1988). Indeed, it is most often exposure to such cues that elicits drug-seeking behavior and relapse in humans (Foltin & Haney, 2000; Grusser, Wrase, Klein, Hermann, Smolka, Ruf, Weber-Fahr, Flor, Mann, Braus, & Heinz, 2004; Shaham, Shalev, Lu, de Wit, & Stewart, 2003). Relative to GTs, STs exhibit increased responding during a test for cue-induced reinstatement, and blockade of dopamine transmission in the NAcC significantly attenuates responding in STs, rendering them more like GTs (Saunders, Yager, & Robinson, 2013). In contrast, when dopamine concentrations in the NAcC are increased via administration of amphetamine, drug-seeking behavior increases in both STs and GTs (Saunders, Yager, & Robinson, 2013). Taken together, these data support a role for dopamine in encoding the incentive motivational value of reward cues and specifically suggest that dopaminergic transmission within the NAcC is a critical part of the neurobiology underlying cue-induced drug-seeking behavior and the propensity to relapse.
When dopamine is released from a neuron into the extracellular space, mechanisms are in place to remove “excess” dopamine from the synapse. The longer dopamine remains in the extracellular space, the more it can interact with neighboring neurons, causing dysregulation of the system. Dopamine is removed from the synapse and relocated back into the presynaptic cell via dopamine transporters (DATs), which exist on the surface of the presynaptic neuron. Dopamine uptake from the extracellular space in the NAcC has been reported to occur more rapidly in STs compared to GTs, and this is thought to be a result of more abundant surface DATs in STs (Singer, Guptaroy, Austin, Wohl, Lovic, Seiler, Vaughan, Gnegy, Robinson, & Aragona, 2016). Certain drugs of abuse, such as cocaine and amphetamine, increase synaptic dopamine concentrations by blocking and inhibiting DATs. Amphetamine also reverses the DATs such that more dopamine is being released into the synapse, further intensifying the effects of the drug. Thus, in individuals with more abundant DATs, amphetamine results in more dopamine being released into the synapse, without efficient removal. In support, administration of amphetamine directly into the NAcC causes slower dopamine reuptake in STs compared to GTs (Singer, Guptaroy, Austin, Wohl, Lovic, Seiler, Vaughan, Gnegy, Robinson, & Aragona, 2016). Additionally, NAcC amphetamine infusions result in an increase in sign-tracking behavior in STs, while behavior in GTs remains unaffected. Thus, the upregulation of DATs in STs compared to GTs appears to contribute to the incentive motivational value of reward cues, and certain drugs of abuse have the ability to amplify these effects. These data further support the notion that dopaminergic transmission within the NAcC contributes to sign-tracking behavior and incentive salience attribution, while goal-tracking behavior is not reliant on these processes.
Synaptic dopamine that is not taken up by the dopamine transporter acts on surrounding cells by binding to dopamine receptors. There are five different types of dopamine receptors, and these receptors can be divided into two families with different functions. The D1 family, composed of the dopamine 1 and 5 receptor, result in excitatory processes within the cell; while the D2 family, containing dopamine receptors 2–4, act in an inhibitory fashion (for review see Keeler, Pretsell, & Robbins, 2014). These receptor subtypes are known to mediate different behaviors and are found in both shared and separate circuitries throughout the brain (for review see Keeler, Pretsell, & Robbins, 2014). The D1 receptor has been associated with mediating phasic dopamine release (Dreyer, Herrik, Berg, & Hounsgaard, 2010), and systemic injection of a D1 antagonist attenuates the acquisition of sign-tracking behavior (Clark, Collins, Sanford, & Phillips, 2013). In recent years, the D3 receptor has also been implicated in motivated behaviors and addiction (Le Foll & Di Ciano, 2015) and has been specifically examined for its role in incentive salience attribution. Systemic administration of a D3 receptor antagonist has no effect on the expression of sign- or goal-tracking behavior (Fraser, Haight, Gardner, & Flagel, 2016). However, systemic administration of compounds that act at both D2 and D3 receptors, either as agonists or antagonists, decrease the conditioned response that had already been learned, whether it is sign- or goal-tracking behavior (Fraser, Haight, Gardner, & Flagel, 2016). These data suggest that D2 receptors, but not D3 receptors, mediate sign- and goal-tracking behaviors, though the mechanism by which this occurs and exactly where in the brain they are acting is still unknown. It should be noted, however, that D2 receptor expression, in the prefrontal cortex as well as the striatum, has been associated with features of addiction in both animals (Briand, Flagel, Garcia-Fuster, Watson, Akil, Sarter, & Robinson, 2008; Flagel, Chaudhury, Waselus, Kelly, Sewani, Clinton, Thompson, Watson, & Akil, 2016) and humans (Asensio, Romero, Romero, Wong, Alia-Klein, Tomasi, Wang, Telang, Volkow, & Goldstein, 2010; Volkow, Wang, Telang, Fowler, Logan, Childress, Jayne, Ma, & Wong, 2006).
Dorsal Striatum
The dorsal striatum, comprised of the caudate and putamen, is also known to play a role in motivation and addiction-related behaviors (B. W. Balleine, Delgado, & Hikosaka, 2007; Volkow, Wang, Fowler, Logan, Jayne, Franceschi, Wong, Gatley, Gifford, Ding, & Pappas, 2002) and has been increasingly recognized for its role in habit formation (for review see Malvaez & Wassum, 2018). Multiple subregions of the dorsal striatum are activated to a greater degree in sign-trackers relative to goal-trackers after presentation of a food- or drug-associated cue (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Yager, Pitchers, Flagel, & Robinson, 2015). One of these subregions, the dorsolateral striatum, has been investigated for its role in incentive salience attribution using the sign-tracker/goal-tracker animal model. When amphetamine is administered directly into this region, the conditioned response of both STs and GTs is amplified, and this is due to increased motivation, not habit formation (DiFeliceantonio & Berridge, 2016). In contrast, however, neither blockade of dopamine signaling within the dorsolateral striatum nor inactivation of this region affects sign-tracking behavior (Fraser & Janak, 2017). This is true with the typical amount of training (i.e., 5 sessions), and persists after prolonged (i.e., 15 sessions) training, when the ventral striatum no longer mediates sign-tracking behavior (Clark, Collins, Sanford, & Phillips, 2013). Thus, although enhanced dopamine signaling in the dorsolateral striatum can increase the incentive motivational value of a Pavlovian cue, making it a stronger motivational magnet, such incentive motivational processes appear to be dependent on dopamine signaling in the ventral and not the dorsal striatum (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b; Fraser & Janak, 2017; Saunders, Yager, & Robinson, 2013).
Midline Thalamus
Paraventricular Nucleus of the Thalamus
One region that has consistently shown the most robust differences in cue-induced neuronal activation between sign- and goal-trackers is the paraventricular nucleus of the thalamus (PVT) (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Yager, Pitchers, Flagel, & Robinson, 2015). The PVT is a midline thalamic nucleus located in an ideal position to influence motivated behaviors, as it acts as an interface to integrate cortical, emotion, and motor networks, and relays this information to the striatum (Kelley, Baldo, Pratt, & Will, 2005). Although this nucleus has been recognized as being part of the motive circuit for over a decade (Kelley, Baldo, Pratt, & Will, 2005), only recently has it gained attention in mediating addiction-related behaviors (Hamlin, Clemens, Choi, & McNally, 2009; James, Charnley, Jones, Levi, Yeoh, Flynn, Smith, & Dayas, 2010; Kuhn, Klumpner, Covelo, Campus, & Flagel, 2017; Matzeu, Kerr, Weiss, & Martin-Fardon, 2016; Matzeu, Weiss, & Martin-Fardon, 2015). When a lesion to the PVT is made prior to Pavlovian training, effectively taking it “off-line,” sign-tracking behavior is amplified, whereas goal-tracking behavior is attenuated (Haight, Fraser, Akil, & Flagel, 2015). When the lesion is made after rats have acquired their conditioned response, the behavior of sign-trackers is not affected (likely due to a ceiling effect); but in GTs, goal-tracking behavior is decreased and sign-tracking behavior increased (Haight, Fraser, Akil, & Flagel, 2015). These data led us to postulate that the PVT may be acting as a “brake” on the attribution of incentive salience to reward cues. Thus, when the PVT is “off-line,” the incentive motivational value of a reward cue is enhanced. In support, inactivation of the PVT prior to a test for cue-induced reinstatement significantly increases drug-seeking behavior in GTs, without affecting sign-trackers (Kuhn, Klumpner, Covelo, Campus, & Flagel, 2017). Thus, it appears that the PVT is a central node that mediates the learning and expression of incentive motivational processes and contributes to individual differences in the propensity to relapse.
Indeed, when correlated neuronal activity is considered between brain regions for each phenotype separately, the PVT is highlighted as a common locus that differentially mediates cue-induced responsivity (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Haight & Flagel, 2014). In STs, cue-induced activity in the PVT is correlated with that in the ventral striatum; whereas in GTs, cue-induced activity in the PVT is correlated with subregions of the prefrontal cortex (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Haight & Flagel, 2014). To further explore the PVT-circuitry that might be differentially regulating the behavior of sign- and goal-trackers, we examined cue-induced neuronal activity selectively in neurons that were directly communicating with the PVT (Haight, Fuller, Fraser, & Flagel, 2017). Relative to controls, both STs and GTs exhibit enhanced cue-induced activity in neurons in the prelimbic cortex that project to the PVT. In contrast, however, STs exhibit enhanced cue-induced activity in subcortical areas, including neurons from the lateral hypothalamus and medial amygdala that project to the PVT, and neurons in the PVT that project to the ventral striatum (Haight, Fuller, Fraser, & Flagel, 2017). These data support the notion that enhanced “bottom-up” processing largely contributes to the sign-tracking phenotype. Next we will briefly review parts of the PVT circuitry—both cortical and subcortical—that we believe are playing a critical role in incentive motivational processes.
PVT Circuitry: Cortical Connections
The prelimbic cortex (PrL) is a subregion of the prefrontal cortex that sends the most dense set of glutamatergic projections to the PVT, while receiving reciprocal glutamatergic projections from the PVT (Li & Kirouac, 2012). The PrL is known to be a critical mediator of both drug- and cue-motivated behaviors, including reinstatement of drug-seeking behavior (for review see Di Ciano, Benham-Hermetz, Fogg, & Osborne, 2007; Di Pietro, Black, & Kantak, 2006; Moorman, James, McGlinchey, & Aston-Jones, 2015). Although cue-induced neuronal activity does not differ between STs and GTs in the PrL (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Yager, Pitchers, Flagel, & Robinson, 2015), correlated activity between the PrL and PVT is evident only in GTs, suggesting that this structure might play a role in differentially mediating the behavioral phenotypes (Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Haight & Flagel, 2014). Indeed, these data, in combination with that reviewed earlier indicating that goal-trackers rely on dopamine-independent cognitive learning processes (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b; Paolone, Angelakos, Meyer, Robinson, & Sarter, 2013; Pitchers, Kane, Kim, Robinson, & Sarter, 2017; Pitchers, Phillips, Jones, Robinson, & Sarter, 2017b), led us to hypothesize that the PrL-PVT pathway may play an important role in exerting cognitive control in GTs. Interestingly, however, we found that, in response to a food-associated cue, STs and GTs engage projections from the PrL to the PVT to the same degree (Haight, Fuller, Fraser, & Flagel, 2017). Thus, since the reward cue is a predictor (i.e., it elicits a conditioned response) for both STs and GTs, we concluded that the PrL-PVT circuit likely encodes the predictive qualities of the cue-CS, and that the enhanced subcortical activity in STs is driving the incentive motivational processes (see Figure 3.2).
PVT Circuitry: Subcortical Connections
As indicated earlier, we examined differences in cue-induced neuronal activity between STs and GTs in a number of subcortical brain regions that are known to project to the PVT, including subnuclei of the amygdala and multiple subregions of the hypothalamus. The medial amygdala (MeA) is one of the regions in which we found greater cue-induced neuronal activity in STs relative to controls in neurons projecting to the PVT (Haight, Fuller, Fraser, & Flagel, 2017). While little is known about the role of the MeA in appetitive-motivated behaviors, early work demonstrated that rats will bar press for electrical stimulation, or self-stimulate the MeA, suggesting that this nucleus does indeed play a role in reward processing (Kane, Coulombe, & Miliaressis, 1991). However, additional work is needed to elucidate the function of the MeA in the circuits that appear to be mediating incentive motivational learning (see Figure 3.2).
Neurons in the lateral hypothalamus (LH) that project to the PVT also show greater cue-induced activity in STs relative to GTs and controls (Haight, Fuller, Fraser, & Flagel, 2017; Figure 3.2). The hypothalamus is known to play an important role in the motive circuit, as it is composed of multiple subregions with various key functions (Kelley, Baldo, Pratt, & Will, 2005). While the dorsomedial nucleus regulates autonomic functions such as blood pressure; the LH mediates aspects of motivation, state-dependent arousal, learning and feeding behaviors (for review see Stuber & Wise, 2016; Tyree & de Lecea, 2017). Thus, it is not surprising that the LH may play an important role in incentive motivational processes. The LH sends orexinergic projections to the PVT (Kirouac, Parsons, & Li, 2005; E. Y. Lee & Lee, 2016; J. S. Lee, Lee, & Lee, 2015), and the role of PVT orexin signaling in addiction-related behaviors has gained increasing attention in recent years (James, Charnley, Levi, Jones, Yeoh, Smith, & Dayas, 2011; Matzeu, Kerr, Weiss, & Martin-Fardon, 2016; Yeoh, Campbell, James, Graham, & Dayas, 2014). For example, blockade of orexin signaling in the PVT prevents cocaine-seeking behavior (Matzeu, Kerr, Weiss, & Martin-Fardon, 2016). In relation, we have found that antagonism of orexin receptors in the PVT attenuates the incentive motivational value of a reward cue and decreases sign-tracking behavior (Campus, Haight, Johnson, Klumpner, Covelo, & Flagel, 2017; Haight, 2016). Thus, orexin signaling in the PVT may be a critical component of the neurobiological mechanisms underlying incentive salience attribution (Figure 3.2).
In addition to examining patterns of cue-induced neuronal activity in regions that send projections to the PVT, we were interested in examining differences in activity in neurons projecting from the PVT to the ventral striatum, a region we know is key in modulating individual differences in reward learning (Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b; Saunders, Yager, & Robinson, 2013). As expected, we found that, relative to controls, STs show enhanced cue-induced activity in neurons projecting from the PVT to the NAc (Haight, Fuller, Fraser, & Flagel, 2017). Importantly, the NAc is a main target of PVT projections (Dong, Li, & Kirouac, 2017), and the PVT can independently elicit dopamine release within the NAc (Parsons, Li, & Kirouac, 2007). Furthermore, this pathway from the PVT to the NAc has been implicated in several addiction-related behaviors, including context-induced reinstatement (Hamlin, Clemens, Choi, & McNally, 2009), long-term effects of cocaine (Neumann, Wang, Yan, Wang, Ishikawa, Cui, Huang, Sesack, Schluter, & Dong, 2016), and opiate dependence (Zhu, Wienecke, Nachtrab, & Chen, 2016). Ongoing work will continue to focus on the role of this pathway in incentive salience attribution, with the hope of elucidating the mechanism by which this circuit influences addiciton-related behaviors.
Taken together, the PVT seems to act as a hub that integrates cortical and subcortical information to guide behavior, but it does so to varying degrees in sign- and goal-trackers. Work thus far suggests that STs rely on enhanced hypothalamic-thalamic-striatal circuitry, whereas the behavior of GTs is primarily mediated by cortical-thalamic processes (see Figure 3.2). Our working hypothesis is that the subcortical processes in STs override the cortical control mechanisms, permitting the attribution of incentive salience to reward cues in an excessive manner.
Hippocampus
While much attention has been focused on the contribution of striatal and thalamic regions in sign- and goal-tracking behavior, other brain regions, such as the hippocampus, have also been implicated in incentive motivational processes (Fitzpatrick, Creeden, Perrine, & Morrow, 2016; Garcia-Fuster, Parsegian, Watson, Akil, & Flagel, 2017). The hippocampus is involved in various types of memory, including that associated with spatial navigation and encoding contextual information (for review see Burgess, Maguire, & O’Keefe, 2002; Martin & Clark, 2007). The hippocampus is often divided anatomically into the ventral (vHPC) and dorsal hippocampus (dHPC), based on differences in function, connectivity, and neurochemistry. For example, it has been shown that lesions of the vHPC affect dopamine activity in the NAc (Lipska, Jaskiw, Chrapusta, Karoum, & Weinberger, 1992), whereas lesions of the dHPC do not (Lipska, Jaskiw, Karoum, Phillips, Kleinman, & Weinberger, 1991). In relation, lesions of the vHPC, but not the dHPC, affect sign-tracking behavior (Fitzpatrick, Creeden, Perrine, & Morrow, 2016). Specifically, lesions of the vHPC prevent the learning of a sign-tracking conditioned response, and concomitantly decrease concentrations of a dopamine metabolite, homovanillic acid (HVA) in the NAc (Fitzpatrick, Creeden, Perrine, & Morrow, 2016). Interestingly, lesions of the vHPC have no effect on sign-tracking behavior after the CR is acquired. Taken together, the ventral hippocampus seems to be another structure involved in incentive motivational learning, presumably via its connections to the ventral striatum (Blaha, Yang, Floresco, Barr, & Phillips, 1997; Floresco, Todd, & Grace, 2001; French & Totterdell, 2002).
Ventral Pallidum
The ventral pallidum (VP) has also been investigated for its role in incentive motivational learning, as it is considered a key component of the reward system and motive circuit (for review see Kalivas & Volkow, 2005; Kelley, Baldo, Pratt, & Will, 2005; Smith, Tindell, Aldridge, & Berridge, 2009). The VP is a primary target of NAcC projections and the mesolimbic dopamine system (Heimer & Wilson, 1975) and is thereby necessary for reward processing and motivated behaviors (Smith, Tindell, Aldridge, & Berridge, 2009). Stimulated activation of the VP causes reward and motivation enhancements (Panagis, Miliaressis, Anagnostakis, & Spyraki, 1995; Smith & Berridge, 2005); whereas lesions of this region decrease the motivation for food and drug rewards (Cromwell & Berridge, 1993; Harvey, Foster, McKay, Carroll, Seyoum, Woods, Grey, Jones, McCane, Cummings, Mason, Ma, Cook, & June, 2002; Shimura, Imaoka, & Yamamoto, 2006). Interestingly, neuronal firing in the VP appears to encode different information in STs and GTs (Ahrens, Meyer, Ferguson, Robinson, & Aldridge, 2016). Neurons in this region respond to the presentation of the cue-CS and the food-US in both phenotypes but with different patterns of activation. In GTs, neuronal activity decreases over the course of cue-CS presentation and increases upon delivery of the food reward. In contrast, in STs, neuronal activity remains high during the entire cue-CS presentation period (Ahrens, Meyer, Ferguson, Robinson, & Aldridge, 2016). Furthermore, neural activity in the VP is positively correlated with the degree of attraction to the food cue. Thus, neural activity in the VP seems to encode the incentive motivational properties of reward cues, presumably via its direct relationship with mesolimbic dopamine transmission in the NAcC (Figure 3.2).
Additional Neurobiological Differences between Sign-Trackers and Goal-Trackers
Thalamic Mast Cell Activity
In addition to specific nuclei of the brain mediating incentive motivational processes, there are also subtler neurobiological processes that appear to contribute to sign-tracking behavior. For example, active mast cells in the thalamus have been associated with sign-tracking behavior (Fitzpatrick & Morrow, 2017b). Mast cells are part of the immune system and reside in the thalamus of the brain until fully developed and functional (Goldschmidt, Hough, Glick, & Padawer, 1984). Once the cells have matured, they can be activated and release various signaling molecules, including monoamines (e.g., dopamine), that can in turn affect surrounding cells (Nautiyal, Ribeiro, Pfaff, & Silver, 2008). Compared to GTs, STs show a greater number of active mast cells in the thalamus, and infusion of a mast cell inhibitor into the lateral ventricle of the brain decreases sign-tracking behavior, without affecting goal-tracking behavior (Fitzpatrick & Morrow, 2017b). Due to the complex nature of mast cells, it is difficult to determine the exact manner by which they are regulating sign-tracking behavior. However, the fact that mast cells can directly affect levels of dopamine (Ronnberg, Calounova, & Pejler, 2012), histamine (Bugajski, Chlap, Gadek-Michalska, Borycz, & Bugajski, 1995; Chikahisa, Kodama, Soya, Sagawa, Ishimaru, Sei, & Nishino, 2013), and corticotrophin-releasing factor (involved in response to stress) (Theoharides, Donelan, Papadopoulou, Cao, Kempuraj, & Conti, 2004), suggests that there are a number of plausible mechanisms by which this might occur.
NMDA Receptors: Effects of Sub-anesthetic Ketamine on Sign-Tracking and Goal-Tracking Behaviors
The involvement of NMDA receptors in sign-tracking behavior has also been investigated through the use of ketamine, a selective NMDA antagonist. Glutamate, one of the main excitatory chemicals in the brain, binds to NMDA receptors which are known to play a role in learning and memory (for review see Castellano, Cestari, & Ciamei, 2001). Several drugs of abuse, such as ketamine, bind to NMDA receptors. However, sub-anesthetic doses of ketamine have been investigated as a treatment for depression (aan het Rot, Collins, Murrough, Perez, Reich, Charney, & Mathew, 2010; Larkin & Beautrais, 2017; Price, Nock, Charney, & Mathew, 2009) and more recently for addiction (Dakwar, Levin, Foltin, Nunes, & Hart, 2014; Krupitsky, Burakov, Romanova, Dunaevsky, Strassman, & Grinenko, 2002). Results thus far have reported decreases in cue-induced cravings and increases in motivation to quit taking drugs in humans following treatment (Dakwar, Levin, Foltin, Nunes, & Hart, 2014; Krupitsky, Burakov, Romanova, Dunaevsky, Strassman, & Grinenko, 2002). The role of sub-anesthetic doses of ketamine in incentive motivational processes was recently investigated using the ST/GT model. Interestingly, a systemic injection of ketamine decreases sign-tracking behavior and increases goal-tracking in STs, without affecting the behavior of GTs (Fitzpatrick & Morrow, 2017a). These results could be a function of altered neurochemical signaling in the prefrontal cortex, a primary target region for ketamine’s effects (Moghaddam, Adams, Verma, & Daly, 1997; Perrine, Ghoddoussi, Michaels, Sheikh, McKelvey, & Galloway, 2014). It is then likely that administration of sub-anesthetic doses of ketamine alters, and possibly strengthens, top-down cortical processes that are otherwise lacking in sign-trackers thus resulting in an increase in goal-tracking behavior (Fitzpatrick & Morrow, 2017a). In support, it was recently reported that STs exhibit an increase in the concentration of extracellular glutamate in the prelimbic cortex of the PFC, as well as in the nucleus accumbens core, during the presentation of a reward cue (Batten, Pomerleau, Quintero, Gerhardt, & Beckmann, 2018). Furthermore, administration of an NMDA receptor antagonist prior to Pavlovian training attenuates the learning of a sign-tracking conditioned response but increases goal-tracking behavior (Chow & Beckmann, 2018). These data suggest that NMDA-mediated glutamatergic transmission, and particularly that within the PFC, contributes to the propensity to attribute incentive salience to reward cues.
Conclusion
The sign-tracker/ goal-tracker model not only captures individual variation in the propensity to attribute incentive motivational value to reward cues but also individual variation in addiction-related behaviors, such as relapse propensity. Using this model, we are able to dissociate the predictive from the incentive value of reward cues and explore the neurobiological mechanisms underlying these distinct associative learning strategies. To date, we know that the behavior of sign-trackers is dopamine-dependent and seemingly reliant on subcortical hypothalamic-thalamic (PVT)-striatal pathways; whereas that of goal-trackers is dependent on cortical cognitive processes. Taken together, we believe it is the imbalance between “top-down” versus “bottom-up” processing that drives the extreme behaviors inherent to each of the phenotypes, including deficits in attentional processing, impulsive behavior (see Meyer and Tripi, this volume) and increased propensity to relapse that are characteristic of sign-trackers.
Acknowledgments
We would like to thank past and present members of the Flagel Lab who have contributed greatly to a number of studies, conclusions, and hypotheses put forth in this chapter. The work included from the Flagel Lab was supported largely by a grant from the National Institute on Drug Abuse (R01DA038599) and training grants (T32DA007821 and T32DA007268).
References
- aan het Rot, M., Collins, K. A., Murrough, J. W., Perez, A. M., Reich, D. L., Charney, D. S., & Mathew, S. J. (2010). Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biological Psychiatry, 67(2), 139–145. doi:10.1016/j.biopsych.2009.08.038.
- Ahrens, A. M., Meyer, P. J., Ferguson, L. M., Robinson, T. E., & Aldridge, J. W. (2016). Neural activity in the ventral pallidum encodes variation in the incentive value of a reward cue. Journal of Neuroscience, 36(30), 7957–7970. doi:10.1523/JNEUROSCI.0736–16.2016.
- Anastasio, N. C., Liu, S., Maili, L., Swinford, S. E., Lane, S. D., Fox, R. G., Hamon, S. C., Nielsen, D. A., Cunningham, K. A., & Moeller, F. G. (2014). Variation within the serotonin (5-HT) 5-HT(2)C receptor system aligns with vulnerability to cocaine cue reactivity. Translational Psychiatry, 4, e369. doi:10.1038/tp.2013.131.
- Asensio, S., Romero, M. J., Romero, F. J., Wong, C., Alia-Klein, N., Tomasi, D., Wang, G. J., Telang, F., Volkow, N. D., & Goldstein, R. Z. (2010). Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse, 64(5), 397–402. doi:10.1002/syn.20741.
- Asplund, C. L., Todd, J. J., Snyder, A. P., & Marois, R. (2010). A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Neuroscience, 13(4), 507–512. doi:10.1038/nn.2509.
- Balleine, B. W., Daw, N. D., & O’Doherty, J. P. (2008). Multiple forms of value learning and the function of dopamine. New York, NY: Academic Press.
- Balleine, B. W., Delgado, M. R., & Hikosaka, O. (2007). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience, 27(31), 8161–8165. doi:10.1523/JNEUROSCI.1554–07.2007.
- Batten, S. R., Pomerleau, F., Quintero, J., Gerhardt, G. A., & Beckmann, J. S. (2018). The role of glutamate signaling in incentive salience: Second-by-second glutamate recordings in awake sprague Dawley Rats. Journal of Neurochemistry, 145(4):276–286 doi:10.1111/jnc.14298.
- Baxter, M. G., & Murray, E. A. (2002). The amygdala and reward. Nature Reviews Neuroscience, 3(7), 563–573. doi:10.1038/nrn875.
- Berridge, K. C. (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl), 191(3), 391–431. doi:10.1007/s00213–006–0578-x.
- Berridge, K. C., & Robinson, T. E. (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Brain Research Review, 28(3), 309–369.
- Berridge, K. C., & Robinson, T. E. (2016). Liking, wanting, and the incentive-sensitization theory of addiction. American Psychology, 71(8), 670–679. doi:10.1037/amp0000059.
- Bindra, D. (1978). How adaptive behavior is produced: a perceptual motivation alternative to response reinforcement Behavior and Brain Sciences (1), 41–91.
- Blaha, C. D., Yang, C. R., Floresco, S. B., Barr, A. M., & Phillips, A. G. (1997). Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. European Journal of Neuroscience, 9(5), 902–911.
- Bolles, R. (1972). Reinforcement, expectancy, and learning. Psychological Review, 79, 394–409. doi:10.1037/h0033120.
- Briand, L. A., Flagel, S. B., Garcia-Fuster, M. J., Watson, S. J., Akil, H., Sarter, M., & Robinson, T. E. (2008). Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology, 33(12), 2969–2980. doi:10.1038/npp.2008.18.
- Bugajski, A. J., Chlap, Z., Gadek-Michalska, A., Borycz, J., & Bugajski, J. (1995). Degranulation and decrease in histamine levels of thalamic mast cells coincides with corticosterone secretion induced by compound 48/80. Inflamm Res, 44 Suppl 1, S50–51.
- Burgess, N., Maguire, E. A., & O’Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron, 35(4), 625–641.
- Campus, P., Accoto, A., Maiolati, M., Latagliata, C., & Orsini, C. (2016). Role of prefrontal 5-HT in the strain-dependent variation in sign-tracking behavior of C57BL/6 and DBA/2 mice. Psychopharmacology (Berl), 233(7), 1157–1169. doi:10.1007/s00213-015-4192-7.
- Campus, P., Haight, J. L., Johnson, A. M., Klumpner, M. S., Covelo, I. R., & Flagel, S. B. (2017, November). The pharmacological antagonism of orexin/hypocretin receptors in the paraventricular nucleus of the thalamus decreases the conditioned reinforcing properties of a reward paired cue in sign-trackers. Paper presented at the Society for Neuroscience, Washington, DC.
- Castellano, C., Cestari, V., & Ciamei, A. (2001). NMDA receptors and learning and memory processes. Current Drug Targets, 2(3), 273–283.
- Chang, S. E., Wheeler, D. S., & Holland, P. C. (2012). Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiol Learn Mem, 97(4), 441–451. doi:10.1016/j.nlm.2012.03.008.
- Chikahisa, S., Kodama, T., Soya, A., Sagawa, Y., Ishimaru, Y., Sei, H., & Nishino, S. (2013). Histamine from brain resident MAST cells promotes wakefulness and modulates behavioral states. PLoS One, 8(10), e78434. doi:10.1371/journal.pone.0078434.
- Childress, A., Ehrman, R., McLellan, A. T., & O’Brien, C. (1988). Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Research Monograph, 81, 74–80.
- Chow, J. J., & Beckmann, J. S. (2018). NMDA receptor blockade specifically impedes the acquisition of incentive salience attribution. Behavioral Brain Research, 338, 40–46. doi:10.1016/j.bbr.2017.10.013.
- Clark, J. J., Collins, A. L., Sanford, C. A., & Phillips, P. E. (2013). Dopamine encoding of Pavlovian incentive stimuli diminishes with extended training. Journal of Neuroscience, 33(8), 3526–3532. doi:10.1523/JNEUROSCI.5119–12.2013.
- Cromwell, H. C., & Berridge, K. C. (1993). Where does damage lead to enhanced food aversion: The ventral pallidum/substantia innominata or lateral hypothalamus? Brain Research, 624(1–2), 1–10.
- Dakwar, E., Levin, F., Foltin, R. W., Nunes, E. V., & Hart, C. L. (2014). The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biological Psychiatry, 76(1), 40–46. doi:10.1016/j.biopsych.2013.08.009.
- Dalley, J. W., Laane, K., Theobald, D. E., Armstrong, H. C., Corlett, P. R., Chudasama, Y., & Robbins, T. W. (2005). Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proceedings of the National Academy of Science U S A, 102(17), 6189–6194. doi:10.1073/pnas.0502080102.
- Daniel, R., & Pollmann, S. (2014). A universal role of the ventral striatum in reward-based learning: Evidence from human studies. Neurobiological Learning Memory, 114, 90–100. doi:10.1016/j.nlm.2014.05.002.
- Danna, C. L., Shepard, P. D., & Elmer, G. I. (2013). The habenula governs the attribution of incentive salience to reward predictive cues. Frontier Human Neuroscience, 7, 781. doi:10.3389/fnhum.2013.00781.
- Davis, M. (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15, 353–375. doi:10.1146/annurev.ne.15.030192.002033.
- Day, J. J., Roitman, M. F., Wightman, R. M., & Carelli, R. M. (2007). Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature Neuroscience, 10(8), 1020–1028. doi:10.1038/nn1923.
- Di Ciano, P., Benham-Hermetz, J., Fogg, A. P., & Osborne, G. E. (2007). Role of the prelimbic cortex in the acquisition, re-acquisition or persistence of responding for a drug-paired conditioned reinforcer. Neuroscience, 150(2), 291–298. doi:10.1016/j.neuroscience.2007.09.016.
- Di Ciano, P., Cardinal, R. N., Cowell, R. A., Little, S. J., & Everitt, B. J. (2001). Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. Journal of Neuroscience, 21(23), 9471–9477.
- Di Pietro, N. C., Black, Y. D., & Kantak, K. M. (2006). Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. European Journal of Neuroscience, 24(11), 3285–3298. doi:10.1111/j.1460–9568.2006.05193.x.
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168.
- Dickinson, A., & Belleine, B. (2002). The role of learning in the operation of motivational systems. In S. Yantis & D. Medin (Eds.), Stevens’ Handbook of Experimental Psychology (pp. 497–533). Hoboken, NJ: John Wiley and Sons, Inc.
- Dietrich, M. O., & Horvath, T. L. (2013). Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neuroscience, 36(2), 65–73. doi:10.1016/j.tins.2012.12.005.
- DiFeliceantonio, A. G., & Berridge, K. C. (2016). Dorsolateral neostriatum contribution to incentive salience: opioid or dopamine stimulation makes one reward cue more motivationally attractive than another. European Journal of Neuroscience, 43(9), 1203–1218. doi:10.1111/ejn.13220.
- Dong, X., Li, S., & Kirouac, G. J. (2017). Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Structure and Function, 222(9), 3927–3943. doi:10.1007/s00429-017-1445-8.
- Dreyer, J. K., Herrik, K. F., Berg, R. W., & Hounsgaard, J. D. (2010). Influence of phasic and tonic dopamine release on receptor activation. Journal of Neuroscience, 30(42), 14273–14283. doi:10.1523/JNEUROSCI.1894–10.2010.
- Ellwood, I. T., Patel, T., Wadia, V., Lee, A. T., Liptak, A. T., Bender, K. J., & Sohal, V. S. (2017). Tonic or phasic stimulation of dopaminergic projections to prefrontal cortex causes mice to maintain or deviate from previously learned behavioral strategies. Journal of Neuroscience, 37(35), 8315–8329. doi:10.1523/JNEUROSCI.1221–17.2017.
- Fitzpatrick, C. J., Creeden, J. F., Perrine, S. A., & Morrow, J. D. (2016). Lesions of the ventral hippocampus attenuate the acquisition but not expression of sign-tracking behavior in rats. Hippocampus, 26(11), 1424–1434. doi:10.1002/hipo.22619.
- Fitzpatrick, C. J., & Morrow, J. D. (2017a). Subanesthetic ketamine decreases the incentive-motivational value of reward-related cues. Journal of Psychopharmacol, 31(1), 67–74. doi:10.1177/0269881116667709.
- Fitzpatrick, C. J., & Morrow, J. D. (2017b). Thalamic mast cell activity is associated with sign-tracking behavior in rats. Brain Behavior Immunity, 65, 222–229. doi:10.1016/j.bbi.2017.05.003.
- Flagel, S. B., Cameron, C. M., Pickup, K. N., Watson, S. J., Akil, H., & Robinson, T. E. (2011a). A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience, 196, 80–96. doi:10.1016/j.neuroscience.2011.09.004.
- Flagel, S. B., Chaudhury, S., Waselus, M., Kelly, R., Sewani, S., Clinton, S. M., Thompson, R. C., Watson, S. J., Jr., & Akil, H. (2016). Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. Proceedings of the National Academy Science U S A, 113(20), E2861–2870. doi:10.1073/pnas.1520491113.
- Flagel, S. B., Clark, J. J., Robinson, T. E., Mayo, L., Czuj, A., Willuhn, I., Akers, C. A., Clinton, S. M., Phillips, P. E., & Akil, H. (2011b). A selective role for dopamine in stimulus-reward learning. Nature, 469(7328), 53–57. doi:10.1038/nature09588.
- Flagel, S. B., & Robinson, T. E. (2017). Neurobiological Basis of Individual Variation in Stimulus-Reward Learning. Current Opinion in Behavioral Science, 13, 178–185. doi:10.1016/j.cobeha.2016.12.004.
- Flagel, S. B., Robinson, T. E., Clark, J. J., Clinton, S. M., Watson, S. J., Seeman, P., Phillips, P. E., & Akil, H. (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology, 35(2), 388–400. doi:10.1038/npp.2009.142.
- Flagel, S. B., Watson, S. J., Robinson, T. E., & Akil, H. (2007). Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl), 191(3), 599–607. doi:10.1007/s00213-006-0535-8.
- Floresco, S. B., Todd, C. L., & Grace, A. A. (2001). Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. Journal of Neuroscience, 21(13), 4915–4922.
- Foltin, R. W., & Haney, M. (2000). Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl), 149(1), 24–33.
- Fraser, K. M., Haight, J. L., Gardner, E. L., & Flagel, S. B. (2016). Examining the role of dopamine D2 and D3 receptors in Pavlovian conditioned approach behaviors. Behavioral Brain Research, 305, 87–99. doi:10.1016/j.bbr.2016.02.022.
- Fraser, K. M., & Janak, P. H. (2017). Long-lasting contribution of dopamine in the nucleus accumbens core, but not dorsal lateral striatum, to sign-tracking. European Journal of Neuroscience, 46(4), 2047–2055. doi:10.1111/ejn.13642.
- French, S. J., & Totterdell, S. (2002). Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. Journal of Comparative Neurology, 446(2), 151–165.
- Fuster, J. M. (2001). The prefrontal cortex—an update: time is of the essence. Neuron, 30(2), 319–333.
- Garcia-Fuster, M. J., Parsegian, A., Watson, S. J., Akil, H., & Flagel, S. B. (2017). Adolescent cocaine exposure enhances goal-tracking behavior and impairs hippocampal cell genesis selectively in adult bred low-responder rats. Psychopharmacology (Berl), 234(8), 1293–1305. doi:10.1007/s00213-017-4566-0.
- Goldschmidt, R. C., Hough, L. B., Glick, S. D., & Padawer, J. (1984). Mast cells in rat thalamus: nuclear localization, sex difference and left-right asymmetry. Brain Research, 323(2), 209–217.
- Grusser, S. M., Wrase, J., Klein, S., Hermann, D., Smolka, M. N., Ruf, M., Weber-Fahr, W., Flor, H., Mann, K., Braus, D. F., & Heinz, A. (2004). Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl), 175(3), 296–302. doi:10.1007/s00213-004-1828-4.
- Haight, J. L. (2016). Elucidating the role of the paraventricular nucleus of the thalamus in cue-motivated behavior. Dissertation, University of Michigan.
- Haight, J. L., & Flagel, S. B. (2014). A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Frontiers in Behavioral Neuroscience, 8, 79. doi:10.3389/fnbeh.2014.00079.
- Haight, J. L., Fraser, K. M., Akil, H., & Flagel, S. B. (2015). Lesions of the paraventricular nucleus of the thalamus differentially affect sign- and goal-tracking conditioned responses. European Journal of Neuroscience, 42(7), 2478–2488. doi:10.1111/ejn.13031.
- Haight, J. L., Fuller, Z. L., Fraser, K. M., & Flagel, S. B. (2017). A food-predictive cue attributed with incentive salience engages subcortical afferents and efferents of the paraventricular nucleus of the thalamus. Neuroscience, 340, 135–152. doi:10.1016/j.neuroscience.2016.10.043.
- Hamlin, A. S., Clemens, K. J., Choi, E. A., & McNally, G. P. (2009). Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. European Journal of Neuroscience, 29(4), 802–812. doi:10.1111/j.1460–9568.2009.06623.x.
- Harvey, S. C., Foster, K. L., McKay, P. F., Carroll, M. R., Seyoum, R., Woods, J. E., 2nd, Grey, C., Jones, C. M., McCane, S., Cummings, R., Mason, D., Ma, C., Cook, J. M., & June, H. L. (2002). The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. Journal of Neuroscience, 22(9), 3765–3775. doi:20026271.
- Heimer, L., & Wilson, R. D. (1975). The subcortical projections of allocortex similarities in the neural associations of the hippocampus, the periform cortex and the neocortex.
- James, M. H., Charnley, J. L., Jones, E., Levi, E. M., Yeoh, J. W., Flynn, J. R., Smith, D. W., & Dayas, C. V. (2010). Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One, 5(9), e12980. doi:10.1371/journal.pone.0012980.
- James, M. H., Charnley, J. L., Levi, E. M., Jones, E., Yeoh, J. W., Smith, D. W., & Dayas, C. V. (2011). Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. International Journal of Neuropsychopharmacology, 14(5), 684–690. doi:10.1017/S1461145711000423.
- Jurado, M. B., & Rosselli, M. (2007). The elusive nature of executive functions: a review of our current understanding. Neuropsychological Review, 17(3), 213–233. doi:10.1007/s11065–007–9040-z.
- Kalivas, P. W., & Volkow, N. D. (2005). The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry, 162(8), 1403–1413. doi:10.1176/appi.ajp.162.8.1403.
- Kane, F., Coulombe, D., & Miliaressis, E. (1991). Amygdaloid self-stimulation: a movable electrode mapping study. Behavioral Neuroscience, 105(6), 926–932.
- Kawa, A. B., Bentzley, B. S., & Robinson, T. E. (2016). Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl), 233(19–20), 3587–3602. doi:10.1007/s00213-016-4393-8.
- Keeler, J. F., Pretsell, D. O., & Robbins, T. W. (2014). Functional implications of dopamine D1 vs. D2 receptors: A ‘prepare and select’ model of the striatal direct vs. indirect pathways. Neuroscience, 282, 156–175. doi:10.1016/j.neuroscience.2014.07.021.
- Kelley, A. E., Baldo, B. A., Pratt, W. E., & Will, M. J. (2005). Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiological Behavior, 86(5), 773–795. doi:10.1016/j.physbeh.2005.08.066.
- Kirouac, G. J., Parsons, M. P., & Li, S. (2005). Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Research, 1059(2), 179–188. doi:10.1016/j.brainres.2005.08.035.
- Koshy Cherian, A., Kucinski, A., Pitchers, K., Yegla, B., Parikh, V., Kim, Y., Valuskova, P., Gurnani, S., Lindsley, C. W., Blakely, R. D., & Sarter, M. (2017). Unresponsive choline transporter as a trait neuromarker and a causal mediator of bottom-up attentional biases. Journal of Neuroscience, 37(11), 2947–2959. doi:10.1523/JNEUROSCI.3499–16.2017.
- Kovacs, K. J. (1998). c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochemistry International, 33(4), 287–297.
- Krupitsky, E., Burakov, A., Romanova, T., Dunaevsky, I., Strassman, R., & Grinenko, A. (2002). Ketamine psychotherapy for heroin addiction: Immediate effects and two-year follow-up. Journal of Substance Abuse Treatment, 23(4), 273–283.
- Kuhn, B. N., Klumpner, M. S., Covelo, I. R., Campus, P., & Flagel, S. B. (2017). Transient inactivation of the paraventricular nucleus of the thalamus enhances cue-induced reinstatement in goal-trackers, but not sign-trackers. Psychopharmacology (Berl), 235(4):999-1014. doi:10.1007/s00213-017-4816-1.
- Larkin, G. L., & Beautrais, A. L. (2017). A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. International Journal of Neuropsychopharmacology, 20(7), 611. doi:10.1093/ijnp/pyx035.
- Le Foll, B., & Di Ciano, P. (2015). Neuronal circuitry underlying the impact of D3 receptor ligands in drug addiction. European Neuropsychopharmacology, 25(9), 1401–1409. doi:10.1016/j.euroneuro.2014.08.017.
- Lee, E. Y., & Lee, H. S. (2016). Dual projections of single orexin- or CART-immunoreactive, lateral hypothalamic neurons to the paraventricular thalamic nucleus and nucleus accumbens shell in the rat: Light microscopic study. Brain Research, 1634, 104–118. doi:10.1016/j.brainres.2015.12.062.
- Lee, J. S., Lee, E. Y., & Lee, H. S. (2015). Hypothalamic, feeding/arousal-related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Research, 1598, 97–113. doi:10.1016/j.brainres.2014.12.029.
- Li, S., & Kirouac, G. J. (2012). Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Structure and Function, 217(2), 257–273. doi:10.1007/s00429-011-0360-7.
- Liljeholm, M., & O’Doherty, J. P. (2012). Contributions of the striatum to learning, motivation, and performance: an associative account. Trends in Cognitive Sciences, 16(9), 467–475. doi:10.1016/j.tics.2012.07.007.
- Lipska, B. K., Jaskiw, G. E., Chrapusta, S., Karoum, F., & Weinberger, D. R. (1992). Ibotenic acid lesion of the ventral hippocampus differentially affects dopamine and its metabolites in the nucleus accumbens and prefrontal cortex in the rat. Brain Research, 585(1–2), 1–6.
- Lipska, B. K., Jaskiw, G. E., Karoum, F., Phillips, I., Kleinman, J. E., & Weinberger, D. R. (1991). Dorsal hippocampal lesion does not affect dopaminergic indices in the basal ganglia. Pharmacology Biochemistry and Behavior, 40(1), 181–184.
- Lovic, V., Saunders, B. T., Yager, L. M., & Robinson, T. E. (2011). Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioral Brain Research, 223(2), 255–261. doi:10.1016/j.bbr.2011.04.006.
- Malvaez, M., & Wassum, K. M. (2018). Regulation of habit formation in the dorsal striatum. Current Opinion in Behavioral Sciences, 20, 67–74. doi:10.1016/j.cobeha.2017.11.005.
- Martin, S. J., & Clark, R. E. (2007). The rodent hippocampus and spatial memory: from synapses to systems. Cellular and Molecular Life Sciences, 64(4), 401–431. doi:10.1007/s00018-007-6336-3.
- Matzeu, A., Kerr, T. M., Weiss, F., & Martin-Fardon, R. (2016). Orexin-A/hypocretin-1 mediates cocaine-seeking behavior in the posterior paraventricular nucleus of the thalamus via orexin/hypocretin receptor-2. Journal of Pharmacology and Experimental Therapeutics, 359(2), 273–279. doi:10.1124/jpet.116.235945.
- Matzeu, A., Weiss, F., & Martin-Fardon, R. (2015). Transient inactivation of the posterior paraventricular nucleus of the thalamus blocks cocaine-seeking behavior. Neuroscience Letters, 608, 34–39. doi:10.1016/j.neulet.2015.10.016.
- Michelsen, K. A., Prickaerts, J., & Steinbusch, H. W. (2008). The dorsal raphe nucleus and serotonin: Implications for neuroplasticity linked to major depression and Alzheimer’s disease. Progress in Brain Research, 172, 233–264. doi:10.1016/S0079–6123(08)00912–6.
- Mihindou, C., Guillem, K., Navailles, S., Vouillac, C., & Ahmed, S. H. (2013). Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biological Psychiatry, 73(3), 271–279. doi:10.1016/j.biopsych.2012.08.011.
- Moghaddam, B., Adams, B., Verma, A., & Daly, D. (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. Journal of Neuroscience, 17(8), 2921–2927.
- Mohammad-Zadeh, L. F., Moses, L., & Gwaltney-Brant, S. M. (2008). Serotonin: A review. Journal of Veterinary Pharmacology and Therapeutics, 31(3), 187–199. doi:10.1111/j.1365–2885.2008.00944.x.
- Montague, P. R., Dayan, P., & Sejnowski, T. J. (1996). A framework for mesencephalic dopamine systems based on predictive Hebbian learning. Journal of Neuroscience, 16(5), 1936–1947.
- Moorman, D. E., James, M. H., McGlinchey, E. M., & Aston-Jones, G. (2015). Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Research, 1628(Pt A), 130–146. doi:10.1016/j.brainres.2014.12.024.
- Nautiyal, K. M., Ribeiro, A. C., Pfaff, D. W., & Silver, R. (2008). Brain mast cells link the immune system to anxiety-like behavior. Proceedings of the National Academy of Sciences U. S. A., 105(46), 18053–18057. doi:10.1073/pnas.0809479105.
- Neumann, P. A., Wang, Y., Yan, Y., Wang, Y., Ishikawa, M., Cui, R., Huang, Y. H., Sesack, S. R., Schluter, O. M., & Dong, Y. (2016). Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacology, 41(9), 2399–2410. doi:10.1038/npp.2016.52.
- Nyberg, L. (2018). Cognitive control in the prefrontal cortex: A central or distributed executive? Scandinavian Journal of Psychology, 59(1), 62–65. doi:10.1111/sjop.12409.
- Panagis, G., Miliaressis, E., Anagnostakis, Y., & Spyraki, C. (1995). Ventral pallidum self-stimulation: A moveable electrode mapping study. Behavioral Brain Research, 68(2), 165–172.
- Paolone, G., Angelakos, C. C., Meyer, P. J., Robinson, T. E., & Sarter, M. (2013). Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. Journal of Neuroscience, 33(19), 8321–8335. doi:10.1523/JNEUROSCI.0709–13.2013.
- Parkinson, J. A., Dalley, J. W., Cardinal, R. N., Bamford, A., Fehnert, B., Lachenal, G., Rudarakanchana, N., Halkerston, K. M., Robbins, T. W., & Everitt, B. J. (2002). Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: Implications for mesoaccumbens dopamine function. Behavioral Brain Research, 137(1–2), 149–163.
- Parkinson, J. A., Olmstead, M. C., Burns, L. H., Robbins, T. W., & Everitt, B. J. (1999). Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. Journal of Neuroscience, 19(6), 2401–2411.
- Parsons, M. P., Li, S., & Kirouac, G. J. (2007). Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. Journal of Comparative Neurology, 500(6), 1050–1063. doi:10.1002/cne.21224.
- Pentkowski, N. S., Duke, F. D., Weber, S. M., Pockros, L. A., Teer, A. P., Hamilton, E. C., Thiel, K. J., & Neisewander, J. L. (2010). Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology, 35(10), 2037–2048. doi:10.1038/npp.2010.72.
- Perrine, S. A., Ghoddoussi, F., Michaels, M. S., Sheikh, I. S., McKelvey, G., & Galloway, M. P. (2014). Ketamine reverses stress-induced depression-like behavior and increased GABA levels in the anterior cingulate: an 11.7 T 1H-MRS study in rats. Progress in Neuropsychopharmacology & Biological Psychiatry, 51, 9–15. doi:10.1016/j.pnpbp.2013.11.003.
- Pitchers, K. K., Kane, L. F., Kim, Y., Robinson, T. E., & Sarter, M. (2017). “Hot” vs. “cold” behavioural-cognitive styles: Motivational-dopaminergic vs. cognitive-cholinergic processing of a Pavlovian cocaine cue in sign- and goal-tracking rats. European Journal of Neuroscience, 46(11), 2768–2781. doi:10.1111/ejn.13741.
- Pitchers, K. K., Phillips, K. B., Jones, J. L., Robinson, T. E., & Sarter, M. (2017b). Diverse roads to relapse: A discriminative cue signaling cocaine availability is more effective in renewing cocaine seeking in goal trackers than sign trackers and depends on basal forebrain cholinergic activity. Journal of Neuroscience, 37(30), 7198–7208. doi:10.1523/JNEUROSCI.0990–17.2017.
- Price, R. B., Nock, M. K., Charney, D. S., & Mathew, S. J. (2009). Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biological Psychiatry, 66(5), 522–526. doi:10.1016/j.biopsych.2009.04.029.
- Ridderinkhof, K. R., van den Wildenberg, W. P., Segalowitz, S. J., & Carter, C. S. (2004). Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognition, 56(2), 129–140. doi:10.1016/j.bandc.2004.09.016.
- Robinson, T. E., & Berridge, K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Brain Research Review, 18(3), 247–291.
- Robinson, T. E., & Flagel, S. B. (2009). Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological Psychiatry, 65(10), 869–873. doi:10.1016/j.biopsych.2008.09.006.
- Robinson, T. E., Yager, L. M., Cogan, E. S., & Saunders, B. T. (2014). On the motivational properties of reward cues: Individual differences. Neuropharmacology, 76 Pt B, 450–459. doi:10.1016/j.neuropharm.2013.05.040.
- Ronnberg, E., Calounova, G., & Pejler, G. (2012). Mast cells express tyrosine hydroxylase and store dopamine in a serglycin-dependent manner. Biological Chemistry, 393(1–2), 107–112. doi:10.1515/BC-2011–220.
- Salamone, J. D., & Correa, M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76(3), 470–485. doi:10.1016/j.neuron.2012.10.021.
- Salin-Pascual, R., Gerashchenko, D., Greco, M., Blanco-Centurion, C., & Shiromani, P. J. (2001). Hypothalamic regulation of sleep. Neuropsychopharmacology, 25(5 Suppl), S21–27. doi:10.1016/S0893–133X(01)00318–9.
- Sarter, M., & Phillips, K. B. (2018). The neuroscience of cognitive-motivational styles: Sign- and goal-trackers as animal models. Behavioral Neuroscience, 132(1):1–12. doi:10.1037/bne0000226.
- Saunders, B. T., O’Donnell, E. G., Aurbach, E. L., & Robinson, T. E. (2014). A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology, 39(12), 2816–2823. doi:10.1038/npp.2014.131.
- Saunders, B. T., & Robinson, T. E. (2010). A cocaine cue acts as an incentive stimulus in some but not others: Implications for addiction. Biological Psychiatry, 67(8), 730–736. doi:10.1016/j.biopsych.2009.11.015.
- Saunders, B. T., & Robinson, T. E. (2011). Individual variation in the motivational properties of cocaine. Neuropsychopharmacology, 36(8), 1668–1676. doi:10.1038/npp.2011.48.
- Saunders, B. T., & Robinson, T. E. (2012). The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience, 36(4), 2521–2532. doi:10.1111/j.1460–9568.2012.08217.x.
- Saunders, B. T., Yager, L. M., & Robinson, T. E. (2013). Cue-evoked cocaine “craving”: Role of dopamine in the accumbens core. Journal of Neuroscience, 33(35), 13989–14000. doi:10.1523/JNEUROSCI.0450–13.2013.
- Schultz, W., Dayan, P., & Montague, P. R. (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599.
- Shaham, Y., Shalev, U., Lu, L., de Wit, H., & Stewart, J. (2003). The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berl), 168(1–2), 3–20. doi:10.1007/s00213–002–1224-x.
- Shimura, T., Imaoka, H., & Yamamoto, T. (2006). Neurochemical modulation of ingestive behavior in the ventral pallidum. European Journal of Neuroscience, 23(6), 1596–1604. doi:10.1111/j.1460–9568.2006.04689.x.
- Shin, L. M., & Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169–191. doi:10.1038/npp.2009.83.
- Singer, B. F., Guptaroy, B., Austin, C. J., Wohl, I., Lovic, V., Seiler, J. L., Vaughan, R. A., Gnegy, M. E., Robinson, T. E., & Aragona, B. J. (2016). Individual variation in incentive salience attribution and accumbens dopamine transporter expression and function. European Journal of Neuroscience, 43(5), 662–670. doi:10.1111/ejn.13134.
- Smith, K. S., & Berridge, K. C. (2005). The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. Journal of Neuroscience, 25(38), 8637–8649. doi:10.1523/JNEUROSCI.1902–05.2005.
- Smith, K. S., Tindell, A. J., Aldridge, J. W., & Berridge, K. C. (2009). Ventral pallidum roles in reward and motivation. Behavioral Brain Research, 196(2), 155–167. doi:10.1016/j.bbr.2008.09.038.
- St Peters, M., Demeter, E., Lustig, C., Bruno, J. P., & Sarter, M. (2011). Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. Journal of Neuroscience, 31(26), 9760–9771. doi:10.1523/JNEUROSCI.1902–11.2011.
- Stewart, J., de Wit, H., & Eikelboom, R. (1984). Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review, 91(2), 251–268.
- Stringfield, S. J., Palmatier, M. I., Boettiger, C. A., & Robinson, D. L. (2017). Orbitofrontal participation in sign- and goal-tracking conditioned responses: Effects of nicotine. Neuropharmacology, 116, 208–223. doi:10.1016/j.neuropharm.2016.12.020.
- Stuber, G. D., & Wise, R. A. (2016). Lateral hypothalamic circuits for feeding and reward. Nature Neuroscience, 19(2), 198–205. doi:10.1038/nn.4220.
- Swinford-Jackson, S. E., Anastasio, N. C., Fox, R. G., Stutz, S. J., & Cunningham, K. A. (2016). Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience, 324, 50–61. doi:10.1016/j.neuroscience.2016.02.052.
- Theoharides, T. C., Donelan, J. M., Papadopoulou, N., Cao, J., Kempuraj, D., & Conti, P. (2004). Mast cells as targets of corticotropin-releasing factor and related peptides. Trends in Pharmacological Science, 25(11), 563–568. doi:10.1016/j.tips.2004.09.007.
- Toates, F. M. (1981). The control of ingestive behaviour by internal and external stimuli—a theoretical review. Appetite, 2(1), 35–50.
- Tomie, A., Di Poce, J., Aguado, A., Janes, A., Benjamin, D., & Pohorecky, L. (2003). Effects of autoshaping procedures on 3H-8-OH-DPAT-labeled 5-HT1a binding and 125I-LSD-labeled 5-HT2a binding in rat brain. Brain Research, 975(1–2), 167–178.
- Tomie, A., Tirado, A. D., Yu, L., & Pohorecky, L. A. (2004). Pavlovian autoshaping procedures increase plasma corticosterone and levels of norepinephrine and serotonin in prefrontal cortex in rats. Behavioral Brain Research, 153(1), 97–105. doi:10.1016/j.bbr.2003.11.006.
- Tyree, S. M., & de Lecea, L. (2017). Lateral hypothalamic control of the ventral tegmental area: Reward evaluation and the driving of motivated behavior. Frontiers in Systems Neuroscience, 11, 50. doi:10.3389/fnsys.2017.00050.
- Vertes, R. P. (1991). A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. Journal of Comparative Neurology, 313(4), 643–668. doi:10.1002/cne.903130409.
- Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Jayne, M., Franceschi, D., Wong, C., Gatley, S. J., Gifford, A. N., Ding, Y. S., & Pappas, N. (2002). “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse, 44(3), 175–180. doi:10.1002/syn.10075.
- Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Logan, J., Childress, A. R., Jayne, M., Ma, Y., & Wong, C. (2006). Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. Journal of Neuroscience, 26(24), 6583–6588. doi:10.1523/JNEUROSCI.1544–06.2006.
- Volkow, N. D., Wise, R. A., & Baler, R. (2017). The dopamine motive system: implications for drug and food addiction. Nature Reviews Neuroscience, 18(12), 741–752. doi:10.1038/nrn.2017.130.
- Waelti, P., Dickinson, A., & Schultz, W. (2001). Dopamine responses comply with basic assumptions of formal learning theory. Nature, 412(6842), 43–48. doi:10.1038/35083500.
- Winstanley, C. A., Dalley, J. W., Theobald, D. E., & Robbins, T. W. (2004). Fractionating impulsivity: Contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology, 29(7), 1331–1343. doi:10.1038/sj.npp.1300434.
- Yager, L. M., Pitchers, K. K., Flagel, S. B., & Robinson, T. E. (2015). Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology, 40(5), 1269–1277. doi:10.1038/npp.2014.314.
- Yager, L. M., & Robinson, T. E. (2013). A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl), 226(2), 217–228. doi:10.1007/s00213–012–2890-y.
- Yeoh, J. W., Campbell, E. J., James, M. H., Graham, B. A., & Dayas, C. V. (2014). Orexin antagonists for neuropsychiatric disease: Progress and potential pitfalls. Frontiers in Neuroscience, 8, 36. doi:10.3389/fnins.2014.00036.
- Zhu, Y., Wienecke, C. F., Nachtrab, G., & Chen, X. (2016). A thalamic input to the nucleus accumbens mediates opiate dependence. Nature, 530(7589), 219–222. doi:10.1038/nature16954.
Figure Legend
Figure 3.1. Rats undergo Pavlovian conditioned approach training whereby an illuminated lever (conditioned stimulus, CS) is inserted into the testing chamber for 8 seconds and immediately upon its retraction a food reward (unconditioned stimulus, US) is delivered to the food cup. At the conclusion of training, rats are characterized as sign-trackers (STs) or goal-trackers (GTs). (a) STs are those who are attracted to and manipulate and engage with the lever-CS during its presentation; whereas, (b) GTs are those who upon lever-CS presentation orient toward the CS, but then immediately go to the food cup to await reward delivery. While the lever-CS is a predictor and elicits a conditioned response for both STs and GTs, only for STs is it also attributed with incentive value and thereby transformed into a “motivational magnet.”
Figure 3.2. A simplified schematic view of the brain circuitry involved in mediating individual variation in cue-motivated behaviors. All of the brain regions listed have been identified as part of the “reward” or “motive” circuits of the brain, and those highlighted in yellow have specifically been investigated for their role in incentive salience attribution. Dotted lines indicate known connections between brain areas and solid lines are proposed circuits regulating the behavior of goal-trackers (red) and sign-trackers (blue). The behavior of goal-trackers is thought to be mediated via “top-down” cognitive control processes; whereas that of sign-trackers is mediated via “bottom-up” subcortical processes. As indicated in the main text, we believe that it is an imbalance between the “top-down” versus “bottom-up” processes that contributes to each of the extreme phenotypes. The following references support the involvement of those nuclei highlighted in yellow (Ahrens, Meyer, Ferguson, Robinson, & Aldridge, 2016; Chang, Wheeler, & Holland, 2012; Danna, Shepard, & Elmer, 2013; DiFeliceantonio & Berridge, 2016; Fitzpatrick, Creeden, Perrine, & Morrow, 2016; Flagel, Cameron, Pickup, Watson, Akil, & Robinson, 2011a; Flagel, Clark, Robinson, Mayo, Czuj, Willuhn, Akers, Clinton, Phillips, & Akil, 2011b; Haight, Fuller, Fraser, & Flagel, 2017; Stringfield, Palmatier, Boettiger, & Robinson, 2017; Yager, Pitchers, Flagel, & Robinson, 2015). Abbreviations: ACC, anterior cingulate cortex; BLA, basolateral amygdala; CeA, central nucleus of the amygdala; CeM, central medial nucleus of the thalamus; dHPC, dorsal hippocampus; DLS, dorsolateral striatum; DMH, dorsomedial nucleus of the hypothalamus; DMS, dorsomedial striatum; DRN, dorsal raphe nucleus; IL, infralimbic cortex; IMD, intermediodorsal nucleus of the thalamus; LA, lateral amygdala; LH, lateral hypothalamus; LHb, lateral habenula; LC, locus coeruleus; MeA, medial nucleus of the amygdala; MHb, medial habenula; NAcC, core of the nucleus accumbens; NAcS, shell of the nucleus accumbens; PrL, prelimbic cortex; PVN, paraventricular nucleus of the hypothalamus; PVT, paraventricular nucleus of the thalamus; VMH, ventromedial nucleus of the hypothalamus; vHPC, ventral hippocampus; VP, ventral pallidum; VTA, ventral tegmental area.


