[Memorandum to Presidential Advisory Council on HIV and AIDS Research Committee Members from Bruce G. Weniger]
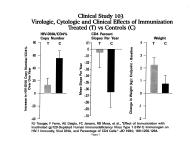
Incentives and Disincentives for Vaccine Development: A Biotech Perspective Manufacturing * Large scale manufacturing capability must be considered when evaluating a vaccine candidate., The technical capabilities for manufacturing large scale cGMP quantities of a well characterized, whole killed, gpl20 depleted product have been established (Figure 5). * Technical hurdles have been overcome for the large-scale production of this product. Page 4
About this Item
- Title
- [Memorandum to Presidential Advisory Council on HIV and AIDS Research Committee Members from Bruce G. Weniger]
- Author
- Weniger, Bruce
- Canvas
- Page 4
- Publication
- 1997-01-10
- Subject terms
- memorandums
- Series/Folder Title
- Government Response and Policy > Presidential > Clinton Administration > Presidential Advisory Council on HIV and AIDS (U.S.) (PACHA) > Meetings and correspondence
- Item type:
- memorandums
Technical Details
- Collection
- Jon Cohen AIDS Research Collection
- Link to this Item
-
https://name.umdl.umich.edu/5571095.0495.212
- Link to this scan
-
https://quod.lib.umich.edu/c/cohenaids/5571095.0495.212/9
Rights and Permissions
The University of Michigan Library provides access to these materials for educational and research purposes, with permission from their copyright holder(s). If you decide to use any of these materials, you are responsible for making your own legal assessment and securing any necessary permission.
Related Links
IIIF
- Manifest
-
https://quod.lib.umich.edu/cgi/t/text/api/manifest/cohenaids:5571095.0495.212
Cite this Item
- Full citation
-
"[Memorandum to Presidential Advisory Council on HIV and AIDS Research Committee Members from Bruce G. Weniger]." In the digital collection Jon Cohen AIDS Research Collection. https://name.umdl.umich.edu/5571095.0495.212. University of Michigan Library Digital Collections. Accessed June 14, 2025.